
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
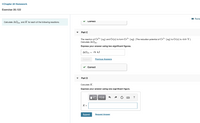
Transcribed Image Text:<Chapter 20 Homework
Exercise 20.122
I Revie
correct
Calculate AG and K for each of the following reactions.
Part C
The reaction of Cr³+ (ag) and Cr(s) to form Cr+ (ag). [The reduction potential of Cr3+ (ag) to Cr(s) is -0.91 V.
Calculate AGn
Express your answer using two significant figures.
AGien = -79 kJ
Submit
Previous Answers
v Correct
Part D
Calculate K.
Express your answer using one significant figure.
?
K =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please explain with correct answerarrow_forwardquestion-arrow_forward23. Consider the following standard reduction potentials. E° (volts) - 2.38 Reduction Half-Reaction Mg*(aq) + 2e→ Mg(s) Zn* (aq) + 2e→ Zn(s) Cu*(aq) + 2e→ Cu*(aq) - 0.76 0.16 Which reaction A-D will proceed spontaneously as written from left to right? a. Mg**(aq) + Zn(s) → Mg(s) + Zn* (aq) b. Mg*(aq) + 2Cu*(aq) → Mg(s) + 2Cu*(aq) c. Zn* (aq) + 2Cu*(aq) → Zn(s) + 2Cu?*(aq) d. Zn(s) + 2Cu*(aq) → Zn" (aq) + 2Cu*(aq) e. None of these will proceed spontaneously.arrow_forward
- Kk.82.arrow_forward1:18 Question 12 of 17 Which pairs of species will spontaneously react? Standard Reduction Potentials 2H*(aq) + 2e → H₂(S) Sn²+ (aq) + 2e → Sn(s) Cd²+ (aq) + 2e → Cd(s) A) Cd and Sn²+ B) Cd with Sn Sn²+ with Cd²+ D) Sn with Cd²+ E) Sn²+ with H* E° (V) 0.00 -0.15 -0.40 Submit Periodic Tablearrow_forwardBalance the following redox reaction if it occurs in acidic solution. What are the coefficients in front of H+ and Mn²+ in the balanced reaction? Fe2+ (aq) + MnO4¹-(aq) A.H+ = 8, Mn2+ = 1 B.H+ = 5, Mn2+ = 1 C.H+ = 8, Mn2+ = 3 D.H+ = 2, Mn2+ = 3 OE.H+ = 3, Mn2+ = 2 + (aq) + Mn²+ (aq) Fe3+arrow_forward
- Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction:Zn2+(aq) + 2Fe2+(aq)Zn(s) + 2Fe3+(aq)Answer: ___kJK for this reaction would be___ (GREATER/LESS) than one.arrow_forwardUse standard reduction potentials to calculate the standard free energy change in kJ for the reaction:Cd2+(aq) + 2Ag(s)Cd(s) + 2Ag+(aq)Answer: kJK for this reaction would be greater/less than one.arrow_forwardConsider the following unbalanced, net ionic, reduction-oxidation expression MnO4- (aq) + Cl2 (g) --> Mn2+ (aq) + ClO- (aq) A) Balance the Mn containing half-reaction in acidic solution. (You do not need to include the state symbols.) MnO4- --> Mn2+ B) Balance the Cl containing half-reaction in acidic solution. (You do not need to include the state symbols.) Cl2 --> ClO-arrow_forward
- Use standard reduction potentials to find the equilibrium constant for the following reaction at 25°C: Mg?*(aq) + Pb(s) =Mg(s) + Pb2+(aq) Enter your answer in scientific notation to one significant digit. K = × 10arrow_forwardplease help me on this one, thank youarrow_forwardHow many grams of zinc, Zn(s) are electroplated out of a solution containing Zn2+ (ag) if the solution is electrolyzed for 22 minutes and 12 seconds at a current of 1.59 amps? Submit your answer with 3 significant figures and do not include units. Answer:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY