
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
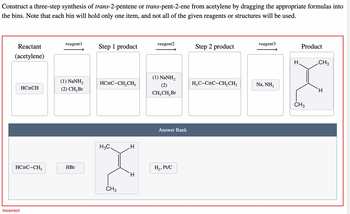
Transcribed Image Text:Construct a three-step synthesis of trans-2-pentene or trans-pent-2-ene from acetylene by dragging the appropriate formulas into
the bins. Note that each bin will hold only one item, and not all of the given reagents or structures will be used.
Reactant
(acetylene)
HC=CH
HC=C-CH,
Incorrect
reagent1
(1) NaNH,
(2) CH₂ Br
HBr
Step 1 product
HC=C-CH,CH3
H3C.
CH3
H
reagent2
(1) NaNH,
(2)
CH₂CH₂ Br
Answer Bank
H₂, Pt/C
Step 2 product
H₂C-C=C-CH₂CH3
reagent3
Na, NH3
H
Product
CH3
CH3
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product.arrow_forwardChoose reagents to convert 2-cyclohexenone to the following compounds. Syntheses may require several steps. Use letters from the table to list reagents in the order used (first at the left). i 1. Li(CH2=CH)2Cu Reagents a 1. Li(CH3)2Cu 2. H3O+ e 1. Li(C6H5)2Cu 2. H3O+ b 1. NaBH4 f CH2l2/Zn(Cu) / ether j 2. H3O+ C NH3 / KOH g 1. CH3MgBr/dry ether k 2 H3O+ d H2NNH2/KOH h HN(CH3)2 HO CH3 a) b) OH N(CH3)2 2. H3O+ (C6H5)3P+-CH2 H₂ over Pd/C KMnO4/H3O+arrow_forwardShow how to convert 4-methyl-1-pentene to the compound shown here. NH₂arrow_forward
- 8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forwardIn both examples below the reactants shown are combined to bring about a nucleophilic substitution (SN1, SN2) and/or elimination (E1, E2) reaction. What is the major reaction that takes place in each case? -Br CH3 CH3CH₂CCH3 ci Nal acetone NaOCH₂CH3 CH3CH₂OH SN2 SN2arrow_forwardWhat is a multi-step synthesis for the following:arrow_forward
- Complete the curved arrow mechanism of the following double elimination reaction when 1,2-dibromopropane is treated with two equivalents of sodium amide and heated in mineral oil. a) Use three curved arrows to show the elimination of the first hydrogen bromide. HN: H 19 H : Br H ↑ b) Use three curved arrows to show the elimination of the second hydrogen bromide. : Br | H H : B H Harrow_forwardConvert 2-pentanol into 2,3-dibromopentane. Draw structures of the starting material (2-pentanol) and final product (2,3-dibromopentane), and show the two reactions needed for this synthesis. Include the structure of the intermediate compound, and the reagents and conditions for each reaction. Then explain why 1,2-dibromopentane would not be a significant product of this synthesis.arrow_forwardDraw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product.arrow_forward
- Predict the major product of this organic reaction: H+ + H₂O ? Specifically, in the drawing area below draw the skeletal ("line") structure of the major product. If there is no reaction, check the No reaction box under the drawing area.arrow_forwardWhat is the mechanism to get from the reactant to the productsarrow_forwardRank the following alkenes in order of increasing rate of hydrogenation. 11 OI<|||< IV < II <|| < |||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY