
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
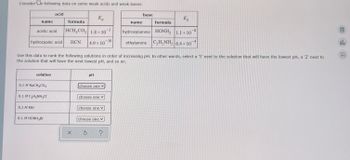
Transcribed Image Text:Considere following data on some weak acids and weak bases:
name
acetic acid
hydrocyanic acid
0.1 M KBr
solution
0.1 M NaCH3CO2
acid
0.1 M C₂H5NH3Cl
0.1 M HONH3Br
formula
HCH₂CO₂ 1.8 x 10-5
HCN
X
Ka
4.9 × 10
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
PH
choose one
choose one
S
choose one
choose one
-10
?
base
name
K₂
formula
hydroxylamine HONH2 1.1 × 10 -8
ethylamine C₂H5NH₂ 6.4 x
×
10
DE!
do
2-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardEach value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. [H+] Acidic = 6.8 × 10-11 [H+] = 1.0 x 10-7 pOH = 1.15 он- pOH = 13.54 pH = 5.65 Basic Answer Bank = 1.2 x 10-5 [OH-] = 3.4 x 10-13 pOH = 7.00 [H+] = 4.1 x 10-3 pH = 9.84 Neutralarrow_forwardCalculate the pH of the solution. Assume 1.0 L of solution. You must identify the type of solution before calculating the pH. Express all pH values to two decimal places. 0.180 moles NaOH and 0.400 moles HC2H3O2 Ka (HC2H3O2) = 1.8 x 10-5arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ba K name formula name formula 5 - 10 hydrocyanic acid HCN 4.9 × 10 ammonia NH3 1.8 × 10 -4 hydrofluoric acid HF 6.8 × 10 aniline C6H5NH2 4.3 × 10¯ - 10 2/5 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaCN PH ✓ choose one 1 (lowest) 0.1 M NH4Br 0.1 M NaNO3 0.1 M KF 2 3 4 (highest) choose one v ☑ ? 00. 18 Ararrow_forwardThe chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid HC103 HI H₂PO4 HF strong or weak? strong strong weak weak species present at 10-6 mol/L or greater when dissolved in water П ☐ 0 X 0,0,... Sarrow_forwardthese are the 3 subparts of a question, please help asaparrow_forward
- The chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid strong or weak? HCIO weak HBrO4 strong H₂PO H₂S species present at 10-6 mol/L or greater when dissolved in water weak ☐ weak ☐ Х 5arrow_forwardConsider the following data on some weak acids and weak bases: acid base Ka K, name formula name formula -4 hydrofluoric acid HF 6.8 × 10 ethylamine C2H;NH, 6.4 × 10 10 4.9 x 10 8. hydrocyanic acid HCN hydroxylamine HONH, 1.1 x 10 Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M HONH3CI choose one 0.1 М KF choose one 0.1 M C2H5NH3Br choose one 0.1 М KBr choose onearrow_forwardacid name formula acetic acid HCH3CO2 1.8 x 10 Consider the following data on some weak acids and weak bases: base formula Kb -5 Ka name ammonia NH₁ 1.8 × 10 -10 hydrocyanic acid HCN 4.9 x 10 pyridine C,HN 1.7x 107 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCI 01 MKCN solution PH choose nee choose one choose one e 0.1 M NH4Br 0.1 M C5H5NHCI choose one earrow_forward
- The chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid HF H₂PO4 HC1 HBrO3 strong or weak? weak weak strong strong species present at 10-6 mol/L or greater when dissolved in water 0 0 0 X 0,0,... Sarrow_forwardThe chemical formulae of some acids are listed in the first column of the table below, and in the second column it says whether each acid is strong or weak. Complete the table. List the chemical formula of each species present at concentrations greater than about 10-6 mol/L when about a tenth of a mole of the acid is dissolved in a liter of water. acid HIO3 HF HBr HC10₂ strong or weak? strong weak strong weak species present at 10-6 mol/L or greater when dissolved in water 0 7 0 0arrow_forwardO ACIDS AND BASES Calculating the pH of a weak base solution 0/3 The base protonation constant ₂ of trimethylamine ((CH³)²N) ¡S 6.31×10¯³. Calculate the pH of a 0.27 M solution of trimethylamine at 25 °C. Round your answer to 1 decimal place. pH = 0 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY