
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Consider three Carnot engines with operating characteristics as follows:
Source temperature
Sink temperature
Efficiency
T1
T3
ea
Carnot engine a
Carnot engine b
Carnot engine c
T1
T2
eb
T2
T3
ec
Show that (1-e,) 3 (1-е.) (1-е.).
Expert Solution
arrow_forward
Step 1
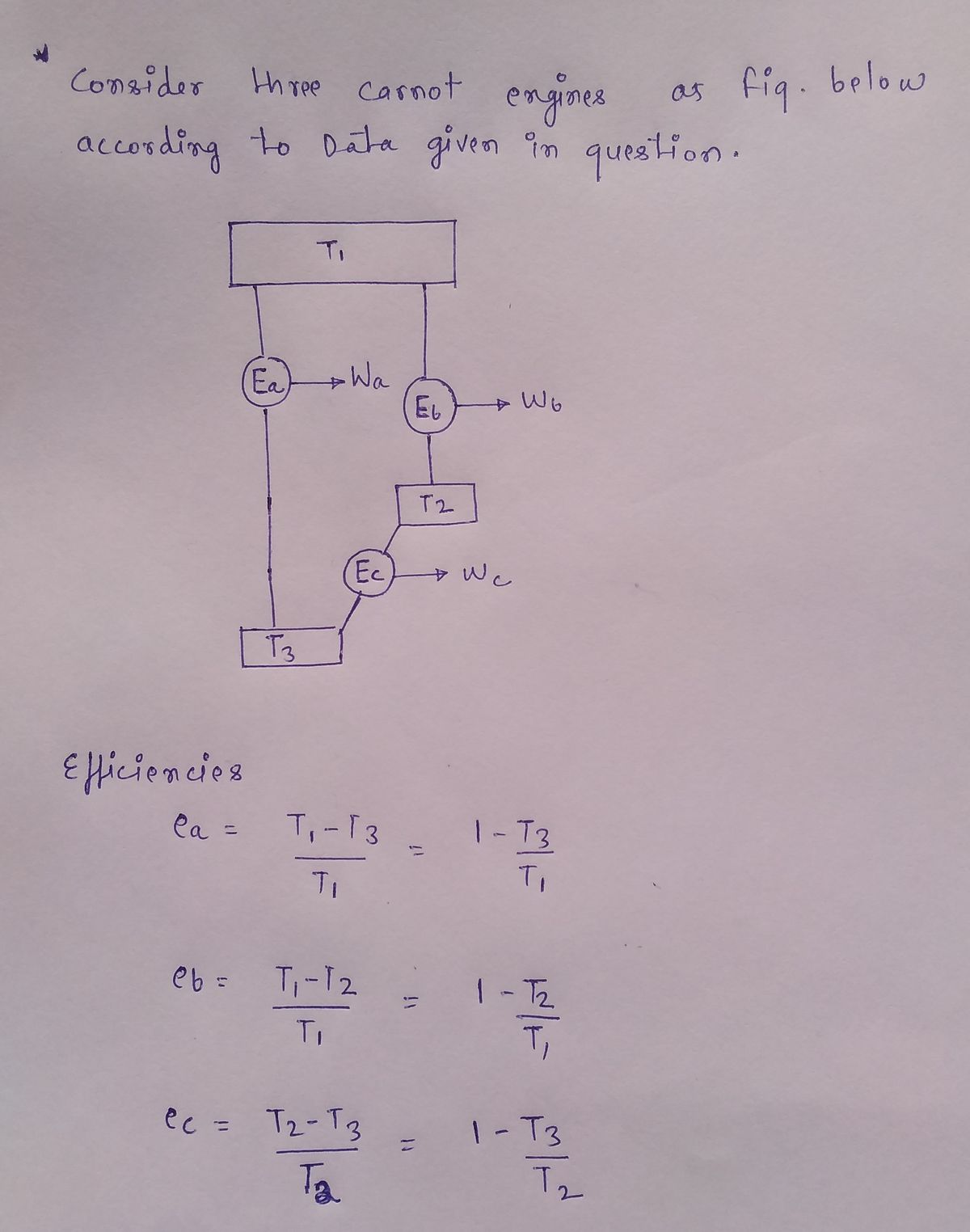
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A 2.00 mol sample of an ideal diatomic gas is taken through a reversible cycle A → B → C → A as shown in the figure below. Process A → B is isothermal expansion. For the cycle, calculate: a) the work done by the gas, b) the heat energy added to the gas, c) the heat energy exhausted by the gas, d) the thermal efficiency, e) the change in entropy of the gas. f) Determine the efficiency of a Carnot engine operating between the same temperature extremes, R = 8.314 J/mol K Data at vertices for the above PV diagram are as follow: PA = 5.00 x 105 Pa, VA = 1.00 x 10 -2 m3, PB = 1.25 x 105 Pa, VB = 4.00 x 10 -2 m3 PC = 1.25 x 105 Pa, VC = 1.00 x 10 -2 m3arrow_forwardExplain the heat transferred from a furnace (Q) to convert the boiler feed water at 25 ° C into superheated steam at 17 bar and 250 ° C.arrow_forwardAn engine has the Lenoir thermodynamic cycle as shown in the figure. Given: 1 mol of ideal gas CP,m = 3.5 R P1 = 2 atm ; P2 = 5 atm V1 = 3000 cm3 ; V3 = 6000 cm3 Find the following: Temperature at (P1, V1), (P2, V2), and (P2, V1) QH and Qc (Note; QH comes from Process 1->2; Proces 2-3 is considered isentropic efficiency of the enginearrow_forward
- Can you provide the step-by-step solution how to get the answers? Steam flows adiabatically through a nozzle from 1517 KPaa, 290 °C, to 965 KPaa. Sketch the pV and Ts diagram and for m=454 gm/sec, Determine delta s, delta u, delta v, delta h, work nonflow and work steady flow. the first three (delta s, delta u, delta v, delta h) were already answered. Can you continue the bold partarrow_forwardDuring the compression stroke of a certain gasoline engine, the pressure increases from 1.00 atm to 19.0 atm. The process is adiabatic and the air-fuel mixture behaves as a diatomic ideal gas. (a) By what factor does the volume change? Vfinal = Vinitial (b) By what factor does the temperature change? Tinal = Tinitial Assume the compression starts with 0.016 mole of gas at 28.5°C. (c) Find the value of Q that characterizes the process. (d) Find the value of AEnt that characterizes the process. (e) Find the value of W that characterizes the process.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY