
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
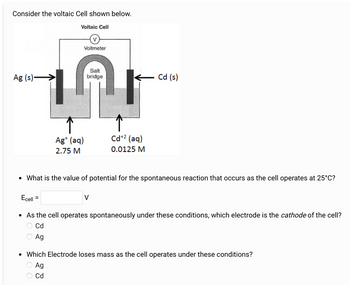
Transcribed Image Text:Consider the voltaic Cell shown below.
Voltaic Cell
V
Voltmeter
Salt
Ag (s)->
bridge
Cd (s)
Ag+ (aq)
2.75 M
Cd+2 (aq)
0.0125 M
• What is the value of potential for the spontaneous reaction that occurs as the cell operates at 25°C?
Ecell
=
• As the cell operates spontaneously under these conditions, which electrode is the cathode of the cell?
Cd
Ag
• Which Electrode loses mass as the cell operates under these conditions?
Ag
Cd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 1 steps with 1 images

Knowledge Booster
Similar questions
- Given: Br2 (1) + 2e 2 Br -1 (aq); 12 (s) + 2e-2 1-¹ (aq); E 0= 0.54 V What is the standard cell potential for the following reaction? 12(s) + 2 Br ¹ (aq) → 21−¹(s) + Br2 (aq) O-1.63 V O-1.63 V O 0.87 V 0.55 V EO = 1.09 V -0.55 Varrow_forwardConsider a voltaic cell where the anode half-reaction isZn(s)---->Zn2+(aq) + 2 e- and the cathode half-reactionis Sn2+ (aq) + 2 e-----> Sn(s). What is the concentration of Sn2+ if Zn2+ is 2.5 x 10-3 M and the cell emf is 0.660 V? Usethe reduction potentials in Appendix E that are reported tothree significant figures. (a) 3.3 x 10-2 M (b) 1.9 x 10-4 M(c) 9.0 x10-3 M (d) 6.9 x 10-4 M (e) 7.6 x 10-3 Marrow_forwardAn electrochemical cell consists of a standard hydrogen electrode and a copper metal electrode. a. What is the potential of the cell at 25°C if the copper electrode is placed in a solution in which = 8.7 x 10-4 M? Ecell = b. The copper electrode is placed in a solution of unknown Cu+ |. The measured potential at 25°C is 0.223 V. What is | Cu²+ |? (Assume Cu²+ is reduced.) [C*] = Cu² 2+ Marrow_forward
- 4 Consider the voltaic cell: Sn(s) | Sn*2(aq) II Fe*3(aq) | Fe*2(aq) a) Write the overall cell reaction. b) Calculate the standard cell potential at 25°C. c) Calculate the change in free-energy of the reaction at 25°C d) What is the maximum work you can obtain from 25.0 g of nickel? e) What is the value of the equilibrium constant of the reaction? f) Which is the stronger oxidizing agent: Sn*2 or Fe*3? Explainarrow_forwardAn electrochemical cell with an overall cell reaction shown below has a standard cell potential of 0.48 V. 2 Al(s) + 3 Mn2*(aq) → 2 Al3+(aq) + 3 Mn(s) i. How many electrons were involved in the overall reaction? ii. What is the AG° of the reaction? iii. What is the Keq of the reaction? iv. What is the new cell potential at 25°C if the initial concentrations of the ions are [Al3+] = 1.50 M, and [Mn2+] = 0.50 M?arrow_forwardWhat is the potential of the reaction?arrow_forward
- O ELECTROCHEMISTRY Designing a galvanic cell from two half-reactions A chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential Br,(1)+2e → 2 Br (aq) =+1.065 V (red 2 H,O(1)+2e Н, 9)+2ОН (аq) -0.83 V ´red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. x10 Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to Yes calculate the cell voltage under standard No conditions? If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 3 significant digits. Explanation Checkarrow_forward35. Calculate the equilibrium constant at 25 °C using standard cell potential data, Sn(s) + CuSO4(aq) = Cu(s) + SnSO4 (aq)arrow_forwardA chemist designs a galvanic cell from the following two half-reactions Ered = +0.96 V NO3(aq) + 4H+ (aq) + 3e¯ → NO(g) + 2H₂O (1) Ered +0.153 V Cu²+ (aq) + e¯ → Cut (aq) The standard cell potential for this galvanic cell is [Select] The standard free energy for this galvanic cell is AG [Select] The equilibrium constant (K) for this galvanic cell is [Select] = V.arrow_forward
- Consider the following voltaic cell at 25°C: Al(s) | Al3*(0.20 M, aq) || Al3+(0.75 M, aq) | Al(s) If the standard reduction potential for the Al3+(aq) /Al(s) redox couple is -1.66 V, what is the overall cell reaction and the cell potential of the voltaic cell shown above? The overall cell reaction is Al3+(0.20 M, ag) Al3*(0.75 M, ag) and Ecell = -0.0113 V. The overall cell reaction is Al3*(0.75 M, aq) AI3+(0.20 M, ag) and Ecell = +0.0113 V. None of these The overall cell reaction is Al3+(0.20 M, aq) Al3*(0.75 M, ag) and Ecell = +0.0113 V. There is no overall cell reaction and Ecell 0.00 V. %3D The overall cell reaction is Al3+(0.75 M, aq) Als*(0.20 M, aq) and Ecell = -0.0113 V.arrow_forwardA voltaic electrochemical cell is constructed using the following reaction. The half-cell components are separated by a salt bridge. 21 (aq) + Cl2(g) → 2(s) + 2CI(aq) Write the reactions that take place at the anode and at the cathode, the direction in which the electrons migrate in the external circuit, and the direction the anions in the salt bridge migrate. Use smallest possible integer coefficients. If a box is not needed, leave it blank. Enter the reaction that takes place at the anode. Include state symbols: Enter the reaction that takes place at the cathode. Include state symbols: In the external circuit, electrons migrate v the Cl2 electrode v the I electrode. Anions migrate v the salt bridge v the Cl2 compartment.arrow_forwardSn(s) | Sn2+ (aq, 1.8 M) || Agt(aq, 0.055 M) | Ag(s) Consider the cell notation of a galvanic cell given above and the following reduction potentials: Sn- (aq) + 2e- → Sn(s) E° = -0.14 V Ag (aq) + e- → Agis) ED=0.80 V 1. The number of electron transferred in the net reaction is [Select] 2. The E is [Select] 3. The value for Q is (Select] 4. The cell potential for the cell is [Select] V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY