
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
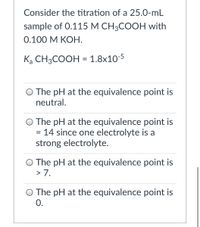
Transcribed Image Text:Consider the titration of a 25.0-mL
sample of 0.115 М СH;COОH with
0.100 М КОН.
Ką CH3COOH = 1.8x10-5
O The pH at the equivalence point is
neutral.
O The pH at the equivalence point is
= 14 since one electrolyte is a
strong electrolyte.
O The pH at the equivalence point is
> 7.
O The pH at the equivalence point is
0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the titration of 40 mL of 0.2 M NH3 with 0.5 M HCI. Calculate the pH after addition of the following amounts of 0.5 M HCI at 25 °C Part A O mL (initial pH) ? νη ΑΣφ Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part B 5 mL ? Πν ΑΣφ Submit Request Answer Part C half-way to equivalence pointarrow_forwardWhich statement explains why the pH at the stoichiometric point in the titration of a weak acid with a strong base is NOT 7.00 (at 25 °C)? O Strong base still is present at the stoichiometric point. O A basic salt is the primary species present at the stoichiometric point. O At the stoichiometric point, a buffer is present. A neutral salt is the primary species present at the stoichiometric point. An acidic salt is the primary species present at the stoichiometric point.arrow_forwardConsider the titration of a 25.0-mL sample of 0.115 M CH3COOH with 0.100 M КОН. Ka CH3COOH = 1.8x10-5 O The pH at the equivalence point is 0. O The pH at the equivalence point is neutral. O The pH at the equivalence point is = 14 since one electrolyte is a strong electrolyte. %3D O The pH at the equivalence point is > 7.arrow_forward
- What is meant by the term "standard" in a standard 0.20 M NaOH solution? What is the difference between an equivalence point and an end point of a titration?arrow_forward11,The equivalence point of titrating of the weak base with the strong acid will be observed at Group of answer choices A, pH>7 B, pH<7 C, pH=pKb D, pH>pKb E, pH=7 12,The titration curve of titrating a diprotic acid with a strong base will have two distinct equivalence points Group of answer choices A, if Ka1<<Ka2 B, if Ka1=Ka2 C, always D, in case of ta strong acid E, if Ka1>>Ka2arrow_forward16.0mL of a 0.750M solution of H>COz are titrated with a 1.0OOM solution of KOH. At what volume of base will the equivalence point be reached? 42.7 mL 12.0 mL O24.0 mL 20.0 mL 21.3 mLarrow_forward
- A 10.0 ml sample of 0.15 M HCIO is titrated with 0.15 M KOH. What is the pH at the equivalence point? K, for HCIO = 4.0 × 10 8. O 2.98 O 11.04 O3.84 O 10.14 O 3.27 O 10.71arrow_forwardTo determine buffer capacity, 40.0mL of solution are titrated with 0.13M NaOH. How many moles of acid were added? initial V- 2.21mL final V- 20.86mL initial pH- 4.32 final pH- 5.61arrow_forwardDilute solutions of the same weak acid (pKą = 4.5) ranging in concentration from 2 × 10-6 M to 2 × 10-2 M (0.002 to 20 mM) are titrated with a strong base that is five times more concentrated than the acid. At low concentrations of a weak acid, how does the concentration of the acid affect the shape of the titration curve? The higher the concentration of the acid, the sharper the inflection at the equivalence point. The lower the concentration of the acid, the sharper the inflection at the equivalence point. The shape of the titration curve is the same, regardless of the acid's concentration. The shape of the titration curve depends only on the pKa of the acid, not its concentration.arrow_forward
- If the pH at the 1/2 equivalence point is 4.19, what would be the Ka?arrow_forward(a) A 25.0 mL sample of 0.175 M methylamine CH3NH2 (Kb = 1.3 x 10-5) is titrated with 0.150 M HBr. Find the initial pH at the ½ equivalence point after 5.0 mL of acid is added the pH at the equivalence point the pH after 5.0 mL acid is added after the equivalence pointarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY