
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Complete these structural formulas by adding enough hydrogens to complete the tetravalence of each carbon. Then write the molecular formula of compound
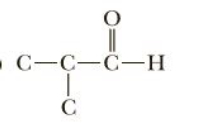
Transcribed Image Text:O C-C-C-H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose you have a car which gets 51 miles per gallon of gasoline in the city. Suppose you take the car to Canada. Then you fill it up at a gas station in Canada. How many kilometers should the car be able to drive on city streets on 1.7 liters of gasoline? (Note: 1 mile = 1.609 km, 1 gallon = 3.78541 L)arrow_forwardHow many hydrogen atoms are in the structure below? C-C-N-Carrow_forwardFor each formula, fill in the eight items listed in the instructions. The Lewis models (at least) should be completed before coming to lab. Be sure to consider the Lewis dot model and the ball-and-stick model as two separate items. The Lewis model should not attempt to indicate geometry. The ball-and-stick model does not need to show multiple bonds or the positions of lone pairs of electrons but should depict molecular geometry. Since ions have a net charge, the issue of polarity is usually unimportant for them. You can skip the polarity analysis for the ions. Please fill in the all blanks.arrow_forward
- PLEASE Hand-draw a Lewis dot and cross diagram of this skeletal compound also is the molecular formula of this compound C8H17NO2 please let me knowarrow_forwardnomenclature of this moleculearrow_forwardSelect all of the following elements that are expected to form compounds with the molecular formula or formula unit consisting of two atoms of one element for every one atom of the other, i.e. AB2 or A2B. O B& H V Ca & Si C & CI Na & S Ca & CIarrow_forward
- Full compound namearrow_forwardDraw a dot structure for a molecule of carbon phosphorus monobromine. The Catom (first drawn if more than one) of carbon phosphorus monobromine would have electrons on top, and numbers in the blanks and O for none). electrons after, electrons before. electrons on bottom of the atomic symbol (use wholearrow_forwardThis are topics that will come in my quiz. Can you give me one example of each? perform calculations using factor-label, unit analysis, conversion factor method (ch. 1) identify compounds as molecular or ionic from formula and electronegativity trends on period table compare, contrast physical properties of ionic vs. molecular compounds write formulas for "complicated" ionic compounds write names of "complicated" ionic compounds polyatomic ions WILL be included in this quiz (polyatomic ions include ammonium, carbonate, chlorate, hydroxide, nitrate, nitrite, phosphate, hydrogen phosphate, sulfate, sulfite...) Identify compounds as acids or basesarrow_forward
- help por favor I just need to draw the moleculearrow_forwardFor each formula, fill in the eight items listed in the instructions. The Lewis models (at least) should be completed before coming to lab. Be sure to consider the Lewis dot model and the ball-and-stick model as two separate items. The Lewis model should not attempt to indicate geometry. The ball-and-stick model does not need to show multiple bonds or the positions of lone pairs of electrons but should depict molecular geometry. Since ions have a net charge, the issue of polarity is usually unimportant for them. You can skip the polarity analysis for the ions. Please fill in the blank!arrow_forwardWhich pair of ions will form a compound of formula M2X?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY