
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
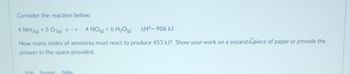
Transcribed Image Text:Consider the reaction below:
4 NH3(g) +502(E) 4 NO(g) + 6 H₂O(g) AH°=-906 kJ
How many moles of ammonia must react to produce 453 kJ? Show your work on a separate piece of paper or provide the
answer in the space provided.
Edit Format Table
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We want to express the equation below for the production of one mole of CH4(g) by specifying, in the equation, the amount of energy required. Al4C3(s) + 12H2O(l) → 4Al(OH)3(s) + 3CH4(g) ∆H = -1763.0 kJ Options: 587.7 kJ on the product side 1763.0 kJ on the reactant side 587.7 kJ on the reactant side 1763.0 kJ on the product sidearrow_forwardThe following chemical reaction is exothermic releasing 283 KJ/mole of heat. CO(g) + ½O2(g) ⟷ CO2(g) How would you increase the amount of product produced from this reaction?arrow_forwardd. e. f. g. h. H₂O Na, NH3 -78°C H₂ Pd/C 1. BH3 - THF 2. NaOH, H₂O2, H₂O HBr H₂ Lindlar's catalyst Tt O Barrow_forward
- The following thermochemical equation is for the reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (C2H2) and water(g). 2CO2(g) + 5H2(g)→C,H2(g) + 4H2O(g) AH= 46.5 kJ When 9.15 grams of carbon dioxide(g) react with excess hydrogen(g), kJ of energy arearrow_forwardPropane reacts with oxygen gas to produce carbon dioxide and water as shown in the reaction below. How much energy in kJ will be released if 500 kg of propane is burned in the air? C3H8 + 50₂ 3CO₂ + 4H₂0 AH = -2221 kJarrow_forward1. Given the following thermochemical data at 25°C and 1 atm pressure, 3 202(g) + 2B(s) → B203(s) AH° =-1264 kJ O3(g) + 2B(s) → B2O3(s) AH° = -1406 kJ determine AH° for the following reaction at 25°C and 1 atm pressure. 203(g) → 302(g)arrow_forward
- Please answer. Thank you for helping mearrow_forwardA typical bike tire has a volume of 150 in³. If a bicycle pump can hold 567 cm³ of air, and you compress it to 350 cm³ with each pump, against an external pressure of 1.0 atm, how much total work (J) would you need to do on the gas in the bicycle pump in order to completely fill the tire? Give your answer as a positive value. Type your numeric answer and submitarrow_forwardConsider the following reaction: 2 Mg + O22 MgO AH xn=-1203 kJ Calculate the amount of heat (in kJ) associated with complete reaction of 97.2 grams of Mg. O-3862 kJ O-4812 kJ O-601.5 kJ -2406 kJ O-1203 kJ 13arrow_forward
- AH Changes sign when a Click on the process is reverNod screen when you started and its derivative demonstrate that the reaction enthalpy, AH, changes sign when a process is reversed. button within the activity and analyze the relationship between the two reactions that are displayed. The reaction that was on the Consider the reaction H2O(1)→H2O(g), AH = 44.0kJ What will AH be for the reaction if it is reversed? Express your answer with appropriate units. > View Available Hint(s) ? HA Value Unitsarrow_forwardNonearrow_forwardIn the following reaction, how many moles of CH3OH are required to give off 3791 kJ of heat? 2 CH,OH (I) + 3 02 (g) → 2 CO, (g) + 4 H,0(g) AH° = -1280. kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY