
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Consider the reaction 2 S(g) + 3 O2(g) → 2 SO3(g). Using the standard enthalpies of formation listed in Appendix G of your text and the bond energy of 498.4 kJ/mol in molecular oxygen, calculate the average sulfur-oxygen bond energy, in units of kJ/mol, in sulfur trioxide gas. The answer is not 473!
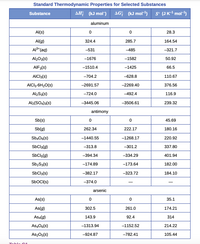
Transcribed Image Text:Standard Thermodynamic Properties for Selected Substances
AH; (kJ mol)
AG; (kJ mol) s° (JKª mol-)
Substance
aluminum
Al(s)
28.3
Al(g)
324.4
285.7
164.54
A"(aq)
-531
-485
-321.7
Al2O3(s)
-1676
-1582
50.92
AIF3(s)
-1510.4
-1425
66.5
AICI3(s)
-704.2
-628.8
110.67
AICI3-6H20(s)
-2691.57
-2269.40
376.56
Al2Sa(s)
-724.0
-492.4
116.9
Al2(SO4)3(s)
-3445.06
-3506.61
239.32
antimony
Sb(s)
45.69
Sb(g)
262.34
222.17
180.16
Sb,Os(s)
-1440.55
-1268.17
220.92
SbCl3(g)
-313.8
-301.2
337.80
SbCls(g)
-394.34
-334.29
401.94
Sb;S3(s)
-174.89
-173.64
182.00
SbCla(s)
-382.17
-323.72
184.10
SBOCI(s)
-374.0
arsenic
As(s)
35.1
As(g)
302.5
261.0
174.21
Asa(g)
143.9
92.4
314
As,O6(s)
-1313.94
-1152.52
214.22
As2Os(s)
-924.87
-782.41
105.44
Telele C1
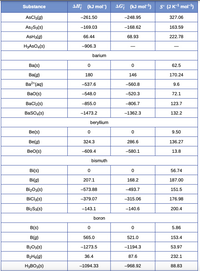
Transcribed Image Text:(kJ mol)
AG; (kJ mol-1)
S° (JK-ª mol-3)
Substance
AsCla(g)
-261.50
-248.95
327.06
As2S3(s)
-169.03
-168.62
163.59
AsH3(g)
66.44
68.93
222.78
H3ASO.(s)
-906.3
barium
Ba(s)
62.5
Ba(g)
180
146
170.24
Ba?"(aq)
-537.6
-560.8
9.6
BaO(s)
-548.0
-520.3
72.1
Bacl2(s)
-855.0
-806.7
123.7
BasO,(s)
-1473.2
-1362.3
132.2
beryllium
Be(s)
9.50
Be(g)
324.3
286.6
136.27
BeO(s)
-609.4
-580.1
13.8
bismuth
Bi(s)
56.74
Bi(g)
207.1
168.2
187.00
BizO3(s)
-573.88
-493.7
151.5
BiCla(s)
-379.07
-315.06
176.98
BizSa(s)
-143.1
-140.6
200.4
boron
B(s)
5.86
B(g)
565.0
521.0
153.4
B203(s)
-1273.5
-1194.3
53.97
B2He(g)
36.4
87.6
232.1
H3BO3(s)
-1094.33
-968.92
88.83
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For which of the following four compounds is the lattice energy most exothermic? CsCl MgCl2 AlCl3 NaClarrow_forwardCombustion reactions involve reacting a substance with oxygen. When compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. Using the enthalpies of combustion for C₂H₂ (-1300. kJ/mol), C₂H6 (-1560. kJ/mol), and H₂ (-286 kJ/mol), calculate AH for the reaction C₂H₂(g) + 2H₂(g) → C₂H6 (9) ΔΗ - Submit Answer Try Another Version Titem attempt remaining Cengage Learning Cengage Technical Support Previous Next> Email Instructor Save and Exitarrow_forwardWhat quantity of energy is required to break up one mole of SO2, and do its constituent elements arrow_forward
- Based on the bond energies for the reaction below, what is the enthalpy of the reaction? HC≡CH (g) + 5/2 O₂ (g) → 2 CO₂ (g) + H₂O (g)arrow_forward1. Draw Lewis structures for ozone and for dioxygen. Using the data given below, qualitatively compare the bond enthalpies, bond orders, and bond lengths of these two compounds. O₂ (g) 20 (g) AH" = +498 kJ O(g) + O₂(g) →O, (g) AH-105 kJarrow_forwardA certain element X forms two compounds with hydrogen, XH3 and X2H4 (H2X–XH2). The total bond enthalpies of XH3(g) and X2H4(g) are 846 kJ/mol and 1341 kJ/mol, respectively. What is the average bond enthalpy of the X–X bond?arrow_forward
- Cyanamide is a compound containing two hydrogen atoms and some amount of C and N. There are a total of 5 atoms in the compound. The products of combustion were found to be CO2, NO2, and H2O. If the enthalpy of combustion for cyanamide is – 671.9 kJ/mol and the enthalpy of formation is 58.8 kJ/mol, what is the chemical formula for cyanamide? ( ΔfH (CO2) = - 393.51 kJ/mole; ΔfH (NO2) = + 33.10 kJ/mole; ΔfH (H2O) = - 241.826 kJ/mole)arrow_forwardHydrazine is an inorganic compound with the chemical formula N₂H4. The reaction of hydrazine with chlorine trifluoride has been used in experimental rocket motors in the reaction below: 3N₂H4 (1) + 4CIF3 (g) → → 3N2 3N2 (g) (g) + 12HF Calculate enthalpy change for the reaction given the following: (1) 2CIF3 + 2NH3N₂ + 6HF + Cl₂ (2) N₂H4 + O₂ → N₂ + 2H₂O (3) 4NH3 + 302 → 2N₂ + 6H₂O (g) Search + 2Cl2 (g) AH = -1196 kJ AH°=-622 kJ AH° = - 1530 kJ Using ethanol as a fuel reduces greenhouse gas emissions by 40-50% when compared directly to ha enthalpies ofarrow_forwardUse the data in Thermodynamic Properties and the bond energy in Bond Energies to determine the average C-C bond energy in benzene (C6H₁). 4.0✔ kJ/mol Calculate the average of a C-C single bond and a C=C double bond using the bond energies, which is the value expected from the Lewis structure. 4.0✔ kJ/mol The larger calculated value using thermodynamic properties is due to the fact that the pi system is delocalized, which makes the molecule more stable than predicted by simply averaging the C-C bonds.arrow_forward
- Use the molar bond enthalpy data in the table to estimate the value of AHin for the equation CC14(g) + 2 F₂ (g) → CF₁ (g) + 2 Cl₂ (g) 2 The bonding in the molecules is shown. ΔΗ +=++· rxn = -CI F-F F-F -C-F CI-CI CI-CI Average molar bond enthalpies (Hbond) Bond Bond O-H C=N 0-0 N-H C-O N-N 0=0 N=N C=O N=N C-C F-F C=C Cl-Cl C=C Br-Br C-H H-H C-F H-F C-Cl H-Cl C-Br H-Br C-N H-S C=N S-S kJ.mol-¹ 464 142 351 502 730 347 615 811 414 439 331 276 293 615 kJ.mol-1 890 390 159 418 945 155 243 192 435 565 431 368 364 225 kJ.mol-¹arrow_forwardUse the bond energies provided for the questionarrow_forwardUsing average bond enthalpies, estimate the enthalpy change for the following reaction: 2CO(g) + O2(g) → 2CO₂(g) AHrxn Bond Bond Energy (kJ/mol) C=O 1072 0=0 498 C=O 732 kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY