
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
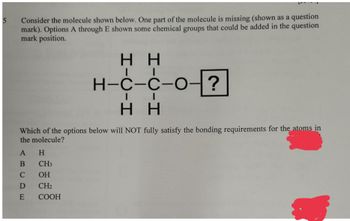
Transcribed Image Text:5
question
Consider the molecule shown below. One part of the molecule is missing (shown as a
mark). Options A through E shown some chemical groups that could be added in the question
mark position.
HH
H-C-C-O-
?
HH
Which of the options below will NOT fully satisfy the bonding requirements for the atoms in
the molecule?
A H
B
C
D
E
CH3
OH
CH₂
COOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 13) The average C−C bond dissociation energy (D) is 350 kJ/mol and the average C=C bond dissociation energy is 728 kJ/mol. Based on these values, which is stronger: a σ or a π bond?arrow_forwardPredicting deviations from ideal bond angles Consider the nitrogen trifluoride (NF3) molecule. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the nitrogen-fluorine bonds? Compared to the ideal angle, you would expect the actual angle between the nitrogen-fluorine bonds to be ... 0 口。 (choose one) (choose one) about the same bigger smallerarrow_forward*:Y:X: :X: The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group (red). In this representation, each Y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of X. There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product arearrow_forward
- < : ed Lewis structure N 0_C_0: H = Br O: the proposed Lewis str Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H chemical symbol, chemical formula or Lewis structure H | HC H | N H- HIC - C C - H Η H HIC C I | H H — — - H Cr O-H Ar :O: O HIC - H C H - H - X boiling point (Choose one) ◊ (Choose one) (Choose one) (Choose one) ✪ Sarrow_forwardCalculate the enthalpy change for the reaction shown below between ammonia and phosgene to produce urea and HCI. Use the bond-dissociation energies listed in the table. Bond H-CI H-N C-CI C-N CI + 2 NH3 AHBDE (kJ/mol) 432 339 397 748 AHrxn = +444 kJ/mol AHrxn= -888 kJ/mol AHrxn= -444 kJ/mol AHrxn= +888 kJ/mol .C. HẠN-“NH, * 2 H-CIarrow_forward
- Lewis Structure of Elements Draw the Lewis structure of elements and determine the number of electrons gained or lost by an element in an ion formation. Lewis Structure Element Compound Formula Compound Formation + [K] [:Br:] KBr Potassium and Bromine K. + ·Br: K. Br: Potassium Bromide Calcium and Nitrogen Lithium and 0xygen Sodium and Phosphorous Aluminum and Sulfurarrow_forwardConsider the phosgene (COC1₂) molecule. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the carbon-chlorine bonds? Compared to the ideal angle, you would expect the actual angle between the carbon-chlorine bonds to be... 0 口。 (choose one) (choose one) about the same bigger smallerarrow_forwardPlease count total number of valence electrons, have lewis structures, resonance structures where applicable, and name the molecular geometry for each central atom.arrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure H : Cl H Nb Cl5 - N | H : Cl: C : Cl: -D - H · Η Cl I C-H I H boiling point ✓ (Choose one) | (highest) 2 3 4 (lowest) (Choose one) (Choose one) ✓arrow_forwardDraw the Lewis symbols for each of the following elements, both before reaction and after reaction to obtain a complete shell: Before reaction. After reaction. P Br Bearrow_forwardConsider the bromate (BrO3) anion. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the bromine-oxygen bonds? Compared to the ideal angle, you would expect the actual angle between the bromine-oxygen bonds to be ... 口。 (choose one) Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY