
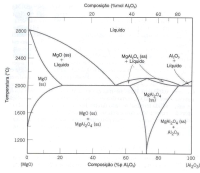
Consider the magnesium oxide-aluminum oxide phase diagram; ss indicates a solid solution. The radii of Mg2 + and Al3 + are 0.072 and 0.053 nm, respectively. (a) There are two eutectic reactions on the diagram; describe them (temperatures, compositions and phases). (b) Spinel, at temperatures below 1000 °C, is a compound with a composition of 50 mol% Al2O3-50 mol% MgO. For higher temperatures, spinel is stable for a range of compositions. What is the temperature of the congruent fusion? (c) What are the main reasons for the low solubility of Al2O3 in MgO below 1400 °C? (d) Maximum non-stoichiometry on the Al2O3-rich side, in the spinel phase field, occurs approximately 2000 °C and corresponds to approximately 82 mol% (92% w) of Al2O3. Determine the type of gaps defect that is produced and the percentage of gaps that exists in this composition.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

- (a) Why is the carbon concentration in austenite (y-Fe) higher than in ferrite (a-Fe)? (b) Determine the volume change accompanied by the transformation of y-Fe (FCC) to a-Fe (BCC) during cooling. Note that the atomic radii for a-Fe, y-Fe, and C are 0.124nm, 0.127mm, and 0.077mm, respectively. (HINT: Because the number of atoms in FCC and BCC differs, consider volume per atom in the calculation of volume change).arrow_forwardCite the phases that are present and the phase compositions for the following alloys: 15 wt% Sn–85 wt% Pb at 100°Carrow_forwardAccording to the following graph, two samples of 1080 steel are cooled from the eutectoid temperature, one at a cooling rate of 250°C/s and the other at a cooling rate of 7.27x10-8 °C/s. Specify the phases obtained and explain their formation from thermodynamic and kinetic perspectives. Also, briefly describe their formation. Draw the microstructure of the phases obtained. Sıcaklık (C) 800 700 600 500 400 300 200 100 0 10 1 T M(başlama) M(% 50) M(% 90) 10 M+O -Otektoid Sıcaklık 10² Zaman (s) % 50 10³ 104 105arrow_forward
- pls immediately help mearrow_forwardAn alloy consisting of completely soluble cadmium (Cd) and zinc (Zn) in the liquid state, but neither of them dissolves in each other in the solid state. the table shown below shows the solidification temperatures for various alloys of cadmium and zinc. 1. Draw the equilibrium diagram according to the information given and data in the table and indicating all important temperature and phases. 2. Find the percentage of each phases and percentage of constituents of the alloy that contain 60 % Zn and at a temperature 300 °C. 3. Find the melting point for the following alloys 20 % Cd, 80% Cd 4. Draw the internal structure, noting the phases of the following alloys A) 30 % Cd at 290 °C b) 60 % Cd at room temperature. % of Zinc in alloy Start of solidification ("C) End of solidification ("C) 0 10 14 20 30 40 50 60 321 290 266 275 293 310 328 345 70 80 90 100 362 390 401 419 266 266 266 266 266 266 266 266 266 266 266 266arrow_forwardConsider 2.0 kg of austenite containing 0.5 wt% C and cooled to just below 727°C (1341°F). (a) What is the proeutectoid phase? (b) How many kilograms each of pearlite, the proeutectoid phase, and cementite form?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





