
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
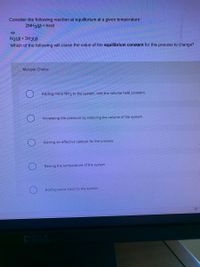
Transcribed Image Text:Consider the following reaction at equilibrium at a given temperature:
2NH3(g) + heat
N2(g) + 3H2(9)
Which of the following will cause the value of the equilibrium constant for this process to change?
Multiple Choice
Adding more NH3 to the system, with the volume held constant.
Increasing the pressure by reducing the volume of the system.
Adding an effective catalyst for the process.
Raising the temperature of the system.
Adding some neon to the system.
POLL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction at equilibrium: 2H₂S (g) 2H₂(g) + S₂ (g) What would be the direction of the above reaction if we increase the concentration of H₂? The reaction will proceed (shift) to the left The reaction would not be affected. The reaction will proceed (shift) to the right.arrow_forwardA vessel at 481°C contains a mixture of 0.147 atm of hydrogen, 0.134 atm of iodine, and 1.003 atm of hydrogen iodide. Is the system at equilibrium? H2(g) + I2(g) = 2HI(g) Кр = 65.2 at 481°C Need to know the volume of the container before deciding. No, therefore the reverse reaction will occur to establish equilibrium. No, therefore the forward reaction will occur to establish equilibrium. Yes. Need to know the initial pressures of all substances before deciding.arrow_forwardHydrogen fluoride is produced by reacting hydrogen with fluorine according to the following equation H₂ (g) + F2 (g) → 2 HF (g) Please use the following information to answer the next question. A stress that would shift the equilibrium towards the products would be to O add HF(g) O remove H₂(g) O decrease the volume of the reaction vessel O decrease the temperature of the reaction vessel AH = -542.2 kJarrow_forward
- Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 104 at a certain temperature. If a solid sample of NH.SH decomposes, what will the equilibrium concentration of NH3 be? NH:SH(s) = NH3(g) + H2S(g) 1 2 3 NEXT > Based on the given values, set up ICE table in order to determine the unknown. NH.SH(s) NH:(g) H2S(g) Initial (M) Change (M) Equilibrium (M) RESET 1.2 x 104 +x +2x -2x 1.2 x 104 + x 1.2 x 104 - x 1.2 x 104 + 2x 1.2 x 104- 2xarrow_forwardIf you decrease the volume of a system containing the following reaction at equilibrium, identify which way will the equilibrium will shift and explain why? 2NO(g) + Cl 2 (g) ↔ 2NOCl(g)arrow_forwardThe equilibrium constant for the following reaction is 0.18 at a set temperature. Find the equilibrium concentrations if the initial concentration of PCl3 is 0.225 mol/L and the initial concentration of Cl2 is 0.150 mol/L. PCI3(g)+CI2(g)=PCI5(g)arrow_forward
- Consider the following system at equilibrium: N₂(g) + 3H₂(g) = 2NH3(g); AH = 92.94 kJ Which of the following changes will shift the equilibrium to the right? Lincreasing the temperature II.decreasing the temperature III increasing the volume IV.decreasing the volume Vremoving some NH3 VLadding some NH3 VII removing some N₂ VIII. adding some N₂ O II, III, V, VIII O I, III, V, VII OI, VI, VIII OLIV, V, VIII O II, IV, V, VIIIarrow_forwardAssume that the following endothermic chemical reaction is at equilibrium. C(s) + H₂O(g) + H₂(g) + CO(g) Which of the following statements is/are CORRECT? 1. Increasing the concentration of H₂(g) will cause the reaction to proceed in the backward direction, increasing the equilibrium concentration of H₂O(g). 2. Decreasing the temperature will cause the reaction to proceed in the forward direction, increasing the equilibrium concentration of CO(g). 3. Increasing the amount of C(s) will cause the reaction to proceed in the forward direction, increasing the equilibrium concentration of CO(g). All statements are correct. 2 only 3 only 1 only 1 & 2 onlyarrow_forwardFor the following reaction 2 SO2(g) + O2(g) <-> 2 SO3(g) + heat If the equilibrium situation is stressed by increasing the volume of the container, the reaction will shift toward a No change b Right or products c Left or reactantsarrow_forward
- For the reaction at equilibrium: 2 H2O (g) +2 Cl2 (g) + energy 4 HCI (g) + O2(g), If a catalyst is added to the reaction mixture. The equilibrium will shift to the left. The equilibrium will shift to the right. The position of the equilibrium will not change.arrow_forwardPhosphorus pentachloride decomposes at higher temperatures. PC15 (8) PCl3(g) + Cl₂(g) An equilibrium mixture at some temperature consists of 5.81 g PCI, 208.23 g/mol 4.86 g PC3, 137.33 g/mol 3.59 g Cl₂, 70.91 g/mol in a 1.00-L flask. If you add 1.31 g of Cl₂, how will the equilibrium be affected and what will the concentration of PCI, be when equilibrium is reestablished? O shift left O shift right Ono shift will occur [PCI5] = mol/Larrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY