
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
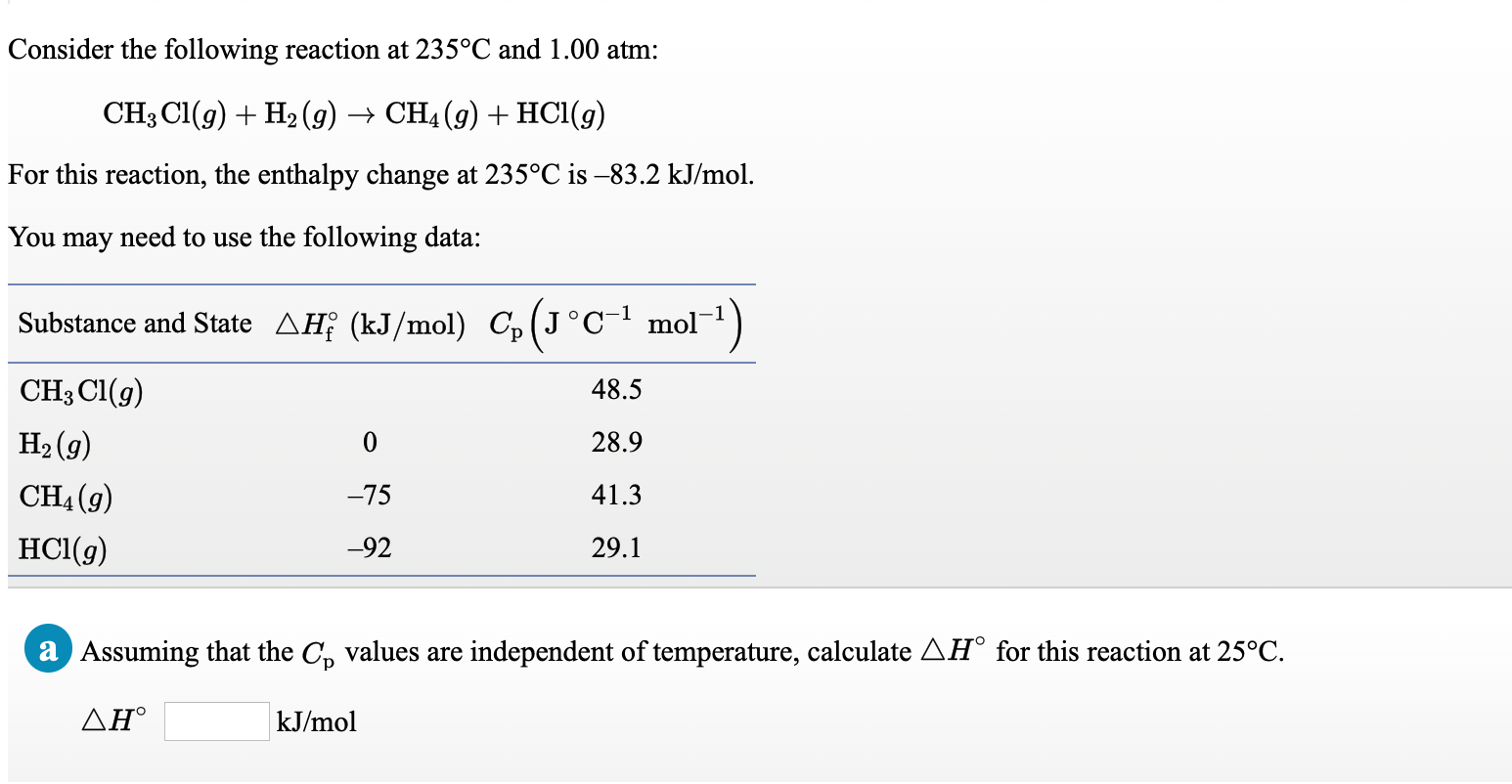
Transcribed Image Text:Consider the following reaction at 235°C and 1.00 atm:
CH3 CI(g) + H2 (9) → CH4 (g) + HC1(g)
For this reaction, the enthalpy change at 235°C is -83.2 kJ/mol.
You may need to use the following data:
Substance and State AĦƐ (kJ/mol) C,(J°Cmol¯1)
CH3 Cl(g)
48.5
H2 (g)
28.9
CH4 (g)
-75
41.3
HCl(g)
-92
29.1
a Assuming that the C, values are independent of temperature, calculate AH° for this reaction at 25°C.
ΔΗ'
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Similar questions
- Another reaction that is used to propel rockets is N2O4(l)+2N2H4(l)3N2(g)+4H2O(g) This reaction has the advantage that neither product is toxic, so no dangerous pollution is released. When the reaction consumes 10.0 g liquid N2O4, it releases 124 kJ of heat. (a) Is the sign of the enthalpy change positive or negative? (b) What is the value of H for the chemical equation if it is understood to be written in molar quantities?arrow_forwardGasohol, a mixture of gasoline and ethanol, C2H5OH, is used as automobile fuel. The alcohol releases energy in a combustion reaction with O2. C2H5OH(l)+3O2(g)2CO2(g)+3H2O(l) If 0.115 g ethanol evolves 3.62 kJ when burned at constant pressure, calculate the combustion enthalpy for ethanol.arrow_forwardEthylene glycol, HOCH2CH2OH, is used as antifreeze. It is produced from ethylene oxide, C2H4O, by the reaction C2H4O(g)+H2O(l)HOCH2CH2OH(l) Use Hesss law to obtain the enthalpy change for this reaction from the following enthalpy changes: 2C2H4O(g)+5O2(g)4CO2(g)+4H2O(l);H=2612.2kJHOCH2CH2OH(l)+52O2(g)2CO2(g)+3H2O(l);H=1189.8kJarrow_forward
- The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per mole of P4S3 at a constant pressure of 1 atm. She wrote the following thermochemical equation: P4S3(s)+8O2(g)P4O10(s)+3SO2(g);H=3651kJ Calculate the standard enthalpy of formation of P4S3, using this students result and the following standard enthalpies of formation: P4O10(s), 3009.9 kJ/mol; SO2(g), 296.8 kJ/mol. How does this value compare with the value given in Appendix C?arrow_forwardThe enthalpy of combustion of diamond is -395.4 kJ/mol. C s, dia O2 g CO2 g Determine the fH of C s, dia.arrow_forwardA sample of benzene, C6H6, weighing 3.51 g was burned in an excess of oxygen in a bomb calorimeter. The temperature of the calorimeter rose from 25.00C to 37.18C. If the heat capacity of the calorimeter and contents was 12.05 kJ/C, what is the value of q for burning 1.00 mol of benzene at constant volume and 25.00C? The reaction is C6H6(l)+152O2(g)6CO2(g)+3H2O(l) Is q equal to U or H?arrow_forward
- Nitrogen gas (2.75 L) is confined in a cylinder under constant atmospheric pressure (1.01 105 pascals). The volume of gas decreases to 2.10 L when 485 J of energy is transferred as heat to the surroundings. What is the change in internal energy of the gas?arrow_forwardA sample of ethanol, C2H5OH, weighing 2.84 g was burned in an excess of oxygen in a bomb calorimeter. The temperature of the calorimeter rose from 25.00C to 33.73C. If the heat capacity of the calorimeter and contents was 9.63 kJ/C, what is the value of q for burning 1.00 mol of ethanol at constant volume and 25.00C? The reaction is C2H5OH(l)+3O2(g)2CO2(g)+3H2O(l) Is q equal to U or H?arrow_forwardWhite phosphorus, P4, ignites in air to produce P4O10. When 3.56 g P4 is burned, 85.8 kJ of thermal energy is evolved at constant pressure. Calculate the combustion enthalpy of P4.arrow_forward
- One of the components of jet engine fuel is n-dodecane, C12H26(), which has a standard enthalpy of combustion of 8080.1 kJ/mol. (a) Write the thermochemical equation for the combustion of n-dodecane. (b) Use the standard enthalpies of formation in Appendix G to calculate the standard enthalpy of formation of n-dodecane.arrow_forwardA 50-mL solution of a dilute AgNO3 solution is added to 100 mL of a base solution in a coffee-cup calorimeter. As Ag2O(s) precipitates, the temperature of the solution increases from 23.78 C to 25.19 C. Assuming that the mixture has the same specific heat as water and a mass of 150 g, calculate the heat q. Is the precipitation reaction exothermic or endothermic?arrow_forwardIn a constant-volume calorimeter, 35.0g of H2cools from 75.3C to25.0C. Calculate w, q, U, and H for the process.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,