
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
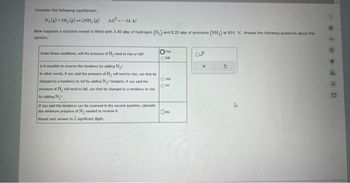
Transcribed Image Text:Consider the following equilibrium:
N₂(g) + 3H₂(g) 2NH, (g) AG=-34. kl
Now suppose a reaction vessel is filled with 3.40 atm of hydrogen (H₂) and 8.20 atm of ammonia (NH,) at 814. "C. Answer the following questions about this
system:
Under these conditions, will the pressure of H₂ tend to rise or fall?
Is it possible to reverse this tendency by adding N₂¹
In other words, If you said the pressure of H₂ will tend to rise, can that be
changed to a tendency to fall by adding Ny? Similarly, if you said the
pressure of Hy will tend to fall, can that be changed to a tendency to rise
by adding N₂7
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of N, needed to reverse it.
Round your answer to 2 significant digits.
Orie
fall
O yes
Ono
7
p
X
5
OG F# S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At a certain temperature, the equilibrium constant K for the following reaction is 1.1: NO3(g) + NO(g) 2 NO₂(g) Use this information to complete the following table. T Suppose a 39. L reaction vessel is filled with 1.3 mol of NO₂- What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. 2 NO₂(9) NO3(g) + NO(g) What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. 2 NO3(g) + 2NO(g) P 4 NO₂(9) There will be very little NO3 and NO. O There will be very little NO₂. ONeither of the above is true. K = K =arrow_forwardConsider the equilibrium N₂O4(g) = 2NO₂(g). At 500 °C, the equilibrium mixture contained 3.86 M nitrogen dioxide gas. Calculate the equilibrium concentration of dinitrogen tetroxide if Kc at this temperature is 0.847. Provide your answer to two places after the decimal and without units. Type your answer...arrow_forwardAt a certain temperature, 0.900 mol SO3 is placed in a 4.50 L container. 2SO3(g)↽−−⇀2SO2(g)+O2(g) At equilibrium, 0.140 mol O2 is present. Calculate ?c.arrow_forward
- O O Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.0074: N,(g) + 0,(g) = 2NO(g) Use this information to complete the following table. Suppose a 22. L reaction vessel is filled with 0.89 mol of N, and 0.89 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and 02. There will be very little NO. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K = ] 2 NO(g) N2(9)+O2(9) What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K =] 2N2(9)+202(g) 4 NO(g) 1. Explanation Check 02021 McGraw-Hill Education All Rights Reserved Terms of Uarrow_forwardKINETICS AND EQUILIBRIUM Using an equilibrium constant to predict the direction of... A chemical engineer is studying the following reaction: 4 HC1(g) + O₂(g) → 2 H₂O(g) + 2Cl₂(g) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.31. The engineer charges ("fills") four reaction vessels with hydrogen chloride and oxygen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel A B с compound HC1 0₂ H₂O Cl₂ HCI 0₂ H₂O Cl₂ HCI 0₂ H₂O C1₂ pressure 5.09 atm 5.63 atm 3.31 atm 4.48 atm 3.91 atm 5.34 atm 3.90 atm 5.07 atm 2.74 atm 5.05 atm 4.48 atm 5.65 atm expected change in pressure O ↑ increase ↑ increase O ↑ increase O ↑ increase O ↑ increase ↑ increase ↑ increase O ↑ increase ↑ increase O ↑ increase ↑ increase ↑…arrow_forwardNitrogen monoxide and water react to form ammonla and oxygen, Ilke this: 4 NO(g) + 6H,O() 4 NH,(g) + 50,(g) Also, a chemist finds that at a certain temperature the equilibrlum mixture of nitrogen monoxide, water, ammonia, and oxygen has the followng composition: compound pressure at equilibrium NO 12.7 atm H,0 94.3 atm NH3 58.1 atm 0, 93.8 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. d. K = x10 Check Explanation ©2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Ac M9 hp & esc % * 00 96 %24arrow_forward
- At a certain temperature, 0.88 mol N, and 2.654 mol H, are placed in a container. N2 (g) + 3H, (g) = 2NH; (g) At equilibrium, there is 0.82 mol NH, present. Determine the number of moles of N, and H, that are present when the reaction is at equilibrium. moles of N, at equilibrium: mol moles of H, at equilibrium: mol | privacy policy terms of use | help about us careers contact us O Ei A Gatewayarrow_forwardSuppose a 500. mL flask is filled with 0.40 mol of N, and 0.10 mol of 0,. The following reaction becomes possible: N, (g) + 0,(g) – 2NO(g) The equilibrium constant K for this reaction is 5.29 at the temperature of the flask. Calculate the equilibrium molarity of N,. Round your answer to two decimal places. OMarrow_forwardNitrogen 2NF3(g)->N2(g) + 3F2(g) When 3.15 mol of NF3 is placed in a 3.00-L container and allowed to come to equilibrium at 900 K, the mixture is found to contain 0.0563 mol of N2. What is the value of K, at this temperature? (R = 0.0821 L'atm-mol¹ K¹). (Hint: Solve for Kc first, then calculate Kp) 4.43 x 10-7 1.8 x 102 1.11 x 10-5 191 x 103 1.07 x 10-5arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY