
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
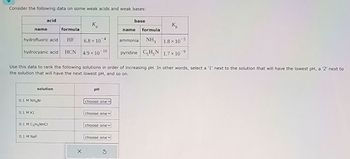
Transcribed Image Text:Consider the following data on some weak acids and weak bases:
base
acid
K
Kb
name
formula
name formula
hydrofluoric acid HF
6.8 x 10
5
hydrocyanic acid HCN 4.9 × 10 10
ammonia NH3 1.8 × 10
pyridine C5H5N 1.7 × 10 9
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
solution
PH
0.1 M NH4Bг
0.1 M KI
0.1 M C5H5NHCI
choose one
choose one
choose onev
0.1 M NaF
choose onev
X
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Complete the table below. Be sure each of your answer entries has the correct number of significant digits. You may assume the temperature is 25 °C. conjugate acid conjugate base Ox10 formula Ka formula к, нсн, со, 1.8 x 10 C,H,ONH 2.1 x 10° CH,NH, 4.4 x 104arrow_forwardConsider the following data on some weak acids and weak bases: base acid Ba K. K, formula name formula name 6- pyridine C5HN 1.7 × 10 -4 hydrofluoric acid HF 6.8 × 10 NH3 - 5 HCH;CO, 1.8 × 10 -5 1.8 × 10 ammonia acetic acid Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH choose one ♥ 0.1 M NaI choose one v 0.1 M NaF 0.1 M KCH3CO2 choose one ♥ 0.1 M C5H5NHB choose onearrow_forwardConsider the following data on some weak acids and weak bases: base acid Ka K name formula name formula hypochlorous acid HCIO 8 3.0 × 10 ethylamine C2H5NH2 6.4 × 10 -4 acetic acid HCH3CO2 1.8 x 10-5 ammonia NH3 1.8 × 10 -5 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution PH choose one 0.1 M NaCH3CO2 0.1 M KI choose one v 0.1 M NH4Bг choose one v 0.1 M C2H5NH3CI choose one varrow_forward
- Hydrogen selenide (H2Se) reacts with water according to the following equation. H2Se + H2O → SeH– + H3O+ Can you identify the acid, base, conjugate acid, and conjugate base in this reaction? Use patterns in the periodic table to explain why the substances you identified acted as the acid and the base in this reaction.arrow_forwardComplete the following table by calculating the missing entries. In each case indicate whether the solution is acidic or basic. PH 5.85 POH 12.45 [H+] ΣΙ 4.60 x 10-10 M M [OH-] M M M 7.30 x 10-1⁰0 M acidic or basic? acidic basic acidic basic acidic basic acidic basicarrow_forwardConsider the following data on some weak acids and weak bases: acid base Ka name formula name formula hypochlorous acid HC1O 3.0 × 10-8 NH3 1.8 x 10 ammonia acețic acid HCH3CO2 1.8 × 10 ethylamine C2H;NH,|6.4×10-4 Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NH4CI choose one 0.1 M Naclo choose one v 0.1 М KсI choose one v 0.1 M C2H5NH3Br choose one varrow_forward
- Consider the following data on some weak acids and weak bases: base acid Κα a Ко name formula name formula hydrofluoric acid 4 HF 6.8 × 10 pyridine CH&N 1.7x10 acetic acid HCH3CO2 1.8 × 10¯¯ methylamine CH3NH2 4.4 × 10¯ -4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCI solution PH ✓ choose one 1 (lowest) 0.1 M CH3NH3Br 0.1 M C5H5NHCI 0.1 M NaF 2 3 4 (highest) choose one v Хarrow_forwardConsider the following data on some weak acids and weak bases: name acetic acid hydrocyanic acid acid solution 0.1 M NaCH3CO2 0.1 M HONH3CI 0.1 M KNO3 0.1 M KCN Ka formula HCH₂CO₂ 1.8 x 10-5 HCN 4.9 × 10 X Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. PH - 10 choose one ŵ choose one ŵ choose one ŵ choose one î S base name Kh formula ethylamine C₂H5NH₂ 6.4 × 107 hydroxylamine HONH, | 1.1 × 10 - 8arrow_forwardEach value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. POH = 13.92 Acidic pH = 12.53 [OH-] = 6.1 × 10-⁹ Basic [H+] = 9.2 x 10-5 pH = 3.20 Answer Bank [OH-] = 7.6 x 10-5 POH Neutral [H+] = 4.61 = 1.0 × 10-7 POH = 7.00 [H+] = 5.7 x 10-11arrow_forward
- Indicate whether the solution is acidic, basic or neutral. Solution pH = 2.4 Acidic Basic Neutral pH = 9.1 %3D РОН 3 6.5 РОН 3D 10.8 1.4 x 104 M NaOH 3.1 х 106 М НСІ [OH] = 1.1 x 10-9 M [H*] = 2.0 x 104 Marrow_forwardPart 1 of 2 Determine the pH of a 0.25 M NH3 solution. Round your answer to 2 decimal places. Note: K₂ for NH3 is 1.78 × 10 b pH = Part 2 of 2 x10 pH = × Determine the pH of a solution that is 0.25 M NH3 and 0.35 M NH4C1. Round your answer to 2 decimal places. Ś x10arrow_forwardWhich of the following pH values indicates greater concentration of base? Group of answer choices pH 9 pH 12 pH 5 pH 1 pH 7arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY