
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
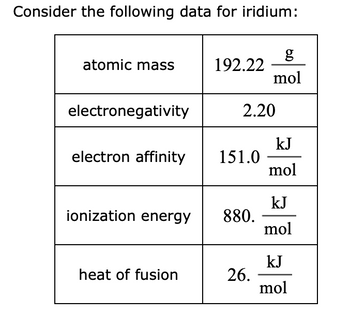
Transcribed Image Text:Consider the following data for iridium:
atomic mass
electronegativity
electron affinity
ionization energy
heat of fusion
192.22
2.20
151.0
880.
g
mol
26.
kJ
mol
kJ
mol
kJ
mol
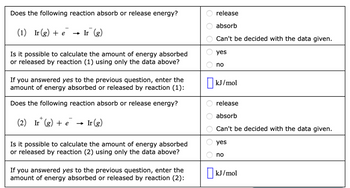
Transcribed Image Text:Does the following reaction absorb or release energy?
(1) Ir (g) + e
→ Ir (g)
Is it possible to calculate the amount of energy absorbed
or released by reaction (1) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (1):
Does the following reaction absorb or release energy?
+
(2) Ir (g) + e Ir (g)
Is it possible to calculate the amount of energy absorbed
or released by reaction (2) using only the data above?
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (2):
OO
release
absorb
Can't be decided with the data given.
yes
no
kJ/mol
release
absorb
Can't be decided with the data given.
yes
no
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following ionic compounds has the largest lattice energy? Select an answer and submit. Forkeyboard navigation, use the up/down arrow keys to select an answer. a CaCl, b. CaO LiBr LICI e LiFarrow_forwardThe energy for the following reaction was measured to be -653.0 kJ/mol. K(g) + Cl(g) → KCl(s). Using this fact and data in the table below, calculate the enthalpy (in kJ/mol) required to separate the ions from the lattice for this reaction: KCl(s) → K⁺(g) + Cl⁻(g).arrow_forwardAluminum(IV) iodide (AII_(4)) cannot be formed. Explain why this is the case by making reference to the energies involved in formation of this compound.arrow_forward
- 6. Place the following elements in order of increasing lattice energy: Na,S KCI MgS CHEM 111 179arrow_forwardUse the data found in the appendix to determine the values for the O-Cl and the O-F bond energies. (heats of formation of OF2 and OCl2 are 24.7 and 80.37 kJ/mol, respectively)arrow_forwardConsider the following data for osmium:arrow_forward
- Choose the related energy for the following reaction: 2 Cs* (g) + O2- (g) → Cs20 (s) electron affinity ionization energy heat of formation lattice energyarrow_forwardMatch lattice energies with following substances: 608, 1030, 2027, 3795, 5278,7492 CsBr, BaCl2, MgO, GaN, Ca3P2, KClarrow_forwardComplete the blanks in each column, as in the first example: Name of the Magnesium Lithium ion Sulfide ion particle atom charge 3+ condensed e [He]3s? [He]2s?2p? [Kr]5s?4d10 configuration atomic Lewis • Mg. :Cl: structurearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY