
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
PLEASE ANSWER WITH DETAIL EXPLANATION CORRECTLY DO NOT COPY FROM OTHER SITE
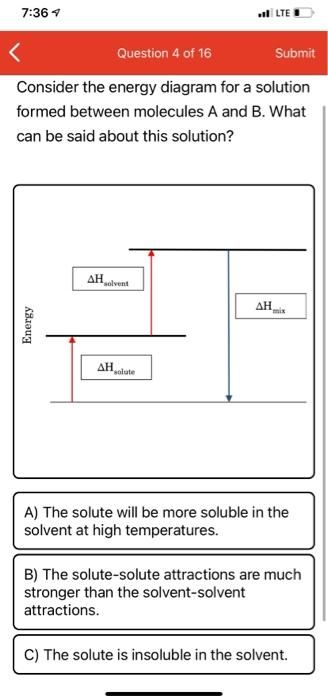
Transcribed Image Text:7:36 7
<
Question 4 of 16
Submit
Consider the energy diagram for a solution
formed between molecules A and B. What
can be said about this solution?
Energy
.
AH
solvent
ΔΗ
solute
LTE
ΔΗ
A) The solute will be more soluble in the
solvent at high temperatures.
B) The solute-solute attractions are much
stronger than the solvent-solvent
attractions.
C) The solute is insoluble in the solvent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the structure of a base. - H,C- Modify the structure to draw the conjugate acid. Add or delete atoms, bonds, charges and non-bonding electrons where appropriate. Select Draw Templates More /||||||| C H H₂C- Ec: Erasearrow_forwardOnly typed explanation otherwise leave itarrow_forwardUse the References to access important values if needed for this question. Including the cis or trans designation, what is the IUPAC name of the following substance? It is not necessary to put cis or trans in italics. Submit Answer Try Another Version 9 item attempts remainingarrow_forward
- . Draw a condensed structure for 2,3-dimethylbutane. Then draw condensed structures for the linear isomer and a branched isomer of that compound, and name those compounds 2,3-dimethylbutane Linear isomer ______________________________Name Branched Isomer ______________________________Namearrow_forwardmechanism please. only typed solutionarrow_forwardWhat type of oxygen-based functional groups may be present in the molecule, based on the IR? Can the molecule contain a/an: aldehyde? [ Select ] ["Yes", "No"] alcohol? [ Select ] ["Yes", "No"] carboxylic acid? [ Select ] ["Yes", "No"] ester? [ Select ] ["Yes", "No"] ether? [ Select ] ["Yes", "No"] ketone? Molecule: C6H12O Degree of unsaturation=1arrow_forward
- Draw an A amino acidarrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. I Don't Know Submitarrow_forwardPlease correct answer and don't use hend raitingarrow_forward
- Head-on overlap is --- than the side on overlaparrow_forwardThis question is from my organic chemistry practice sheetarrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. X H H-C-H H-O-H H 3 Note for advanced students: what we mean by "cutting" the bond here is breaking the bond and attaching H atoms to each dangling end, like this: H H-C-0-H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY