
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Report probl
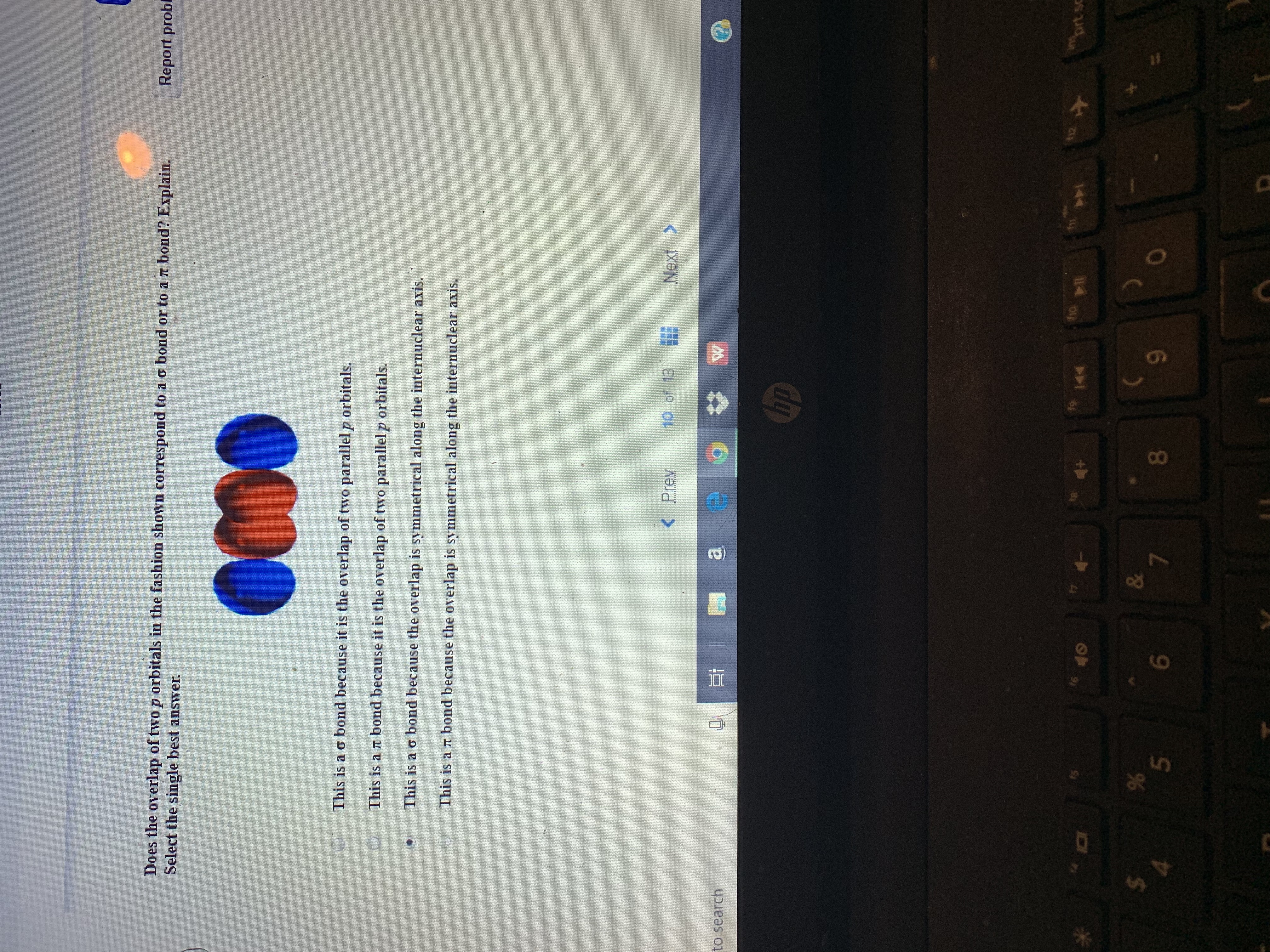
Does the overlap of two p orbitals in the fashion shown correspond to a o bond or to a rt bond? Explain.
Select the single best answer
This is a o bond because it is the overlap of two parallel p orbitals.
This is a r bond because it is the overlap of two parallel p orbitals.
This is a o bond because the overlap is symmetrical along the internuclear axis.
This is a rT bond because the overlap is symmetrical along the interuclear axis.
10 of 13
Next>
<Prev
a
to search
up
prt sc
te
144
f6
&
7
%
5
8
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Nonearrow_forwardDraw the Lewis structure for the H₂CCCH₂ molecule and then answer the following questions. What is the number of o bonds? Number (Enter an integer.) (Enter an integer.) What is the number of bonds? Number What is the ideal H-C-C angle? Click for List What is the ideal C-C-C angle? Click for List What is the hybridization of the central C atom? What is the hybridization of the C atoms that are bonded to the H atoms? Click for List Does this molecule have a planar shape? (i.e. Do all the atoms lie on the same plane?) Click for List degrees (Choose the closest value.) degrees (Choose the closest value.) Click for Listarrow_forwardIf a theoretical atom has: 14 protons 16 electrons 13 neutrons What is the mass number for this atom?arrow_forward
- Pls help ASAP. Pls do all asked questions I BEGarrow_forwardCHEM-025 Chapter 7 Lewis Structures & VSEPR Theory Electron Molecular Steric Bond m n Class Geometry Geometry Sketch Notes Hybridization # angle(s) (Shape) 2 2 0 3 3 | 0 3 2 1 4 4 4 3| 1 4 2| 2 5 5 | 0 1arrow_forward14 Draw the Lewis structure of CH3CCH and then choose the appropriate set of hybridization states for the three central atoms. Your answer choice is independent of the orientation of your drawn structure. + Click to draw a new structure Question 18 of 18 ditional resources A) sp/sp/sp B) sp³/sp/sp C) sp³ / sp² / sp² D) sp²/sp/sp³ E) sp / sp³ / sp³arrow_forward
- Will you explain this problem please?arrow_forwardsee attachedarrow_forwardConsider the molecule shown below, and the pair of energy diagrams, for the next set of questions. Enter the letters corresponding to atoms in the molecule (abc etc). If none of the atoms apply, input none. 1. Which of the atom(s) is/are sp3 hybridized? Submit Answer Tries 0/45 2. Which of the atom(s) is/are sp2 hybridized? Tries 0/45 atom(s) is/are sp hybridized? Tries 0/45 atom(s) is/are represented by the energy diagram on the left? Tries 0/45 Submit Answer 3. Which of the Submit Answer 4. Which of the Submit Answer 5. Which of the Submit Answer atom(s) is/are represented by the energy diagram on the right? Tries 0/45 Post Discussion h Send Feedbackarrow_forward
- What would be the shape of a molecule where the central atom has two nonbonding pairs while also covalently bonded to two other atoms? Group of answer choices bent pyramidal tetrahedral linear T-shapedarrow_forwardNov 14 Draw the Lewis structure of CH₂NOH and then choose the appropriate set of hybridization states for the three central atoms. Your answer choice is independent of the orientation of your drawn structure. C=N HI -H :O- Click to edit molecule itional resources Question 17 of 18 A) sp² / sp² / sp² B) sp/sp/sp² C) sp³ / sp³ / sp³ D) sp² / sp³ / sp E) sp³ / sp 5²arrow_forwardWhich of these statements concerning p-orbitals is false? 1. They consist of two equivalent lobes. 2. They are absent from the first shell of atomic orbitals. 3. They can form & bonds. 4. They only participate in bonding on carbon atoms. 5. They can hold a maximum of two electrons.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY