
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
This problem is (11.16) from a book "
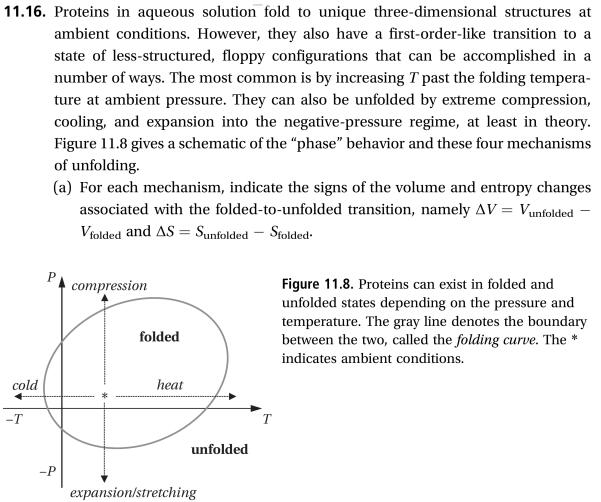
Transcribed Image Text:11.16. Proteins in aqueous solution fold to unique three-dimensional structures at
ambient conditions. However, they also have a first-order-like transition to a
state of less-structured, floppy configurations that can be accomplished in a
number of ways. The most common is by increasing T past the folding tempera-
ture at ambient pressure. They can also be unfolded by extreme compression,
cooling, and expansion into the negative-pressure regime, at least in theory
Figure 11.8 gives a schematic of the "phase" behavior and these four mechanisms
of unfolding.
(a) For each mechanism, indicate the signs of the volume and entropy changes
associated with the folded-to-unfolded transition, namely AV Vunfolded
Vfolded and AS Sunfolded Sfolded
Figure 11.8. Proteins can exist in folded and
unfolded states depending on the pressure and
temperature. The gray line denotes the boundary
between the two, called the folding curve. The
indicates ambient conditions.
compression
folded
heat
cold
-T
unfolded
expansion/stretching

Transcribed Image Text:(b) Explain why the cooling and stretching mechanisms might be difficult
or impossible to realize in an experiment. Hint: what would happen if the
solution contained no proteins?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- What is the Gibbs phase rule for the general system?arrow_forwardMULTIPLE CHOICE -The answer is one of the options below please solve carefully and circle the correct option Please write clear .arrow_forwardThe discipline dealing with material distribution between coexisting phases is called Select one: O a. Fluid dynamics O b. Physical chemistry O c. Civil engineering O d. Phase-equilibrium thermodynamicsarrow_forward
- In a 2-component, 2-phase system, write Raoult's law for component 1 and component 2, and derive the equation that relates Bubble temperature to Bubble point, and the equation that relates Dew temperature to Dew point.arrow_forwardi am confused on where you are getting these equations. is f--- friction? why is it 64/row VD/viscosity? are there other units at play here? i am not seeing how they cancel out to become Pa/marrow_forwardPlease detail explain the physical meaning of parameters of Fick’s first law and second.arrow_forward
- It proposes values for plotting the 6 different viscosity models (Dilatant, Newtonian, Psudoplastic, Plastic Bingham, Psudoplastic Bingham, Dilatant Bingham), all with the help of the formula:All this with the help of matlab. I need help plz i don't realy now how to use this.arrow_forwardA mixture of three components (A, B, and C) enters a separation process.The three components appear in the distillate with variable composi-tion. In contrast, only B and C appear in the bottom. Write a proper set ofmaterial balance equationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The