
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Consider carrying out two elementary, non-reversible reactions
in parallel in a batch reactor:
Write down the three differential equations describing time-evolution of the
concentrations of species A, B, and C. If you want to solve the equations, what do you need
in addition to the three differential equations
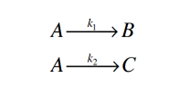
Transcribed Image Text:AB
A-₂ C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider a batch reactor in which the following liquid phase reaction is occurring:A → BThe reaction is first order with rate constant k and is exothermic. The batch reactor isa jacketed vessel and the jacket is maintained at a temperature Tj by an electrical heater.Develop a mathematical model that describes the dynamics of the concentrations of speciesA and B and the reactor temperature T.arrow_forwardA chemical engineer is studying the rate of this reaction. He fills a reaction vessel with and measures its concentration as the reaction proceeds. Here's a graph of his data: 0.050.10.150.200.020.040.060.080.10 Use this graph to answer the following questions: What is the half life of the reaction? Round your answer to significant digits. Suppose the rate of the reaction is known to be first order in . Calculate the value of the rate constant . Round your answer to significant digits. Also be sure you include the correct unit symbol. Predict the concentration of in the engineer's reaction vessel after seconds have passed. Assume no other reaction is important, and continue to assume the rate is first order in . Round your answer to significant digits.arrow_forwardA mixture is the overhead of a distillation column and it is liquefied in the overhead total condenser which operates at 50 psig. From a simulation, can you explain why it is not a good idea to put a control valve on the overhead line that goes to the total condenser? Note: -no additional info needed for this problem. - be as detailed as possible in the explaination. Thanks!arrow_forward
- 6.19 Consider the following cascade connection of isothermal reactors. A first-order reaction A Boccurs in both reactors, with reaction rate constant k. The volumes of liquid in the reactors, V, are constant and equal; the flow rates Fo, Fi, F2 and R are constant. Assume constant physical properties and a negligible time delay for the recycle line. Fo. Co R, C F1, C1 Figure E6.19 (a) Write a mathematical model for this process. (b) Derive a transfer function model relating the output con- centration of A, C2 to the inlet concentration of A, C (c) Verify that, in the limit of no recycle (R-0), the transfer function derived in (b) is equivalent to the transfer function of the two tanks connected in series. (d) Show that when k- 0 and a very large recycle flow rate is used (i.e., the limit as Ro), the transfer function derived in (b) becomes the transfer function of a single tank that has the volume equal to 2V and a gain of one. Hint: Recognize that FRF2 and FoF2arrow_forwardPlease answer correctlyarrow_forward13) The following gas-phase complex reactions follow elementary rate laws A+ 2B-C 2A+ C D RB=WBa=k'C(CB-CBS)α = KCB CB and take place isothermally in an isothermal membrane reactor. An equal molar feed of A and B enter the reactor, and species C is able to diffuse through the membrane. Pressure drop is significant in the reactor. Set up the equations that you would need to plot and analyze the molar flow rates of each species and conversion of species A as a function of volume. Include all equations that you would need to enter into Polymath except for explicit equations defining constants (i.e., vo = 1 L/min, etc.) or initial and final conditions. A species: A,B,C,D MB: =rA dFA dFB=B dFc CFD =rc-RC V dv dv CH =rD Rate laws: -VA=KACACB -VA = KACACC N Net Rate laws: rj = Σ Relative Rates: VA=VB = C=D Fj olchiometry: C = CTo FP7 = C=C TO + T=FA+FB+FC+FD FT PO FJ =Cj=CTO! FT isothermal essure Drop: d (P/Po) - dy -- a T FT Ap dw dw Hold this for each specie 2y 24 TO FTO X=> RC = 7arrow_forward
- You are designing a reactor for a process that has the following decomposition reaction: A →D + 2F The rate law for this reaction is unknown and must be determined by an experiment. Since the reaction takes place in the liquid phase, you choose to build a CSTR reactor. You allow the reactor to fill to a certain volume, V, and then adjust the inlet and outlet volumetric flow rates to equal values, vo, to allow the system to reach steady state. Once the system is at steady state you measure the outlet concentration, CA. The residence time (t = V/v.) is increased by turning off the outlet flow, which allows the volume to increase, then setting the outlet flow to its original value and waiting until steady state is reached. You measure the concentration again and repeat this procedure multiple times to obtain the following table. Measurement 1 2 (ii) 234 in 3 5 Residence Time (min) 15.0 38.0 100 300 1200 Concentration (mol/L) 1.50 1.25 1.00 0.75 0.50 The inlet concentration (containing…arrow_forwardA liquid reactant stream (1 mol/liter) passes through two mixed flow reactors in a series. The concentration of A in the exit of the first reactor is 0.5 mol/liter. Find the concentration in the exit stream of the second reactor. The reaction is second-order with respect to A and V2/V1 = 2. reactor designarrow_forward7.What is a similarity between the Chernobyl reactor and more modern reactor designs? A.In both systems, the fission reaction can be run at extremely low power levels without the reactor shutting down completely. B.In both systems, the fission reactor is encased in an underground building, which will prevent leakage of radioactive material. C.In both systems, the fission reaction is controlled by control rods and is cooled by cycling water through the system. D.In both systems, the fission reaction is controlled by cooling water, which is circulated through the system via pumps that draw power from the reactor.arrow_forward
- Reaction Engineering The first order, reversible reaction A ↔ B + 2Cis taking place in a membrane reactor. Pure A enters the reactor, and B diffuses through the membrane. Unfortunately, some of reactant A also diffuses through the membrane. Other information of the reaction in the membrane reactor is shown in the image attached. Please formulate the differential equations that can be used to solve for the flow rates of species A, B, and C as a function of reactor volume and the flow rates of A and B through the membranearrow_forwardA chemical engineer is studying the rate of this reaction. He fills a reaction vessel with and measures its concentration as the reaction proceeds. Here's a graph of his data: 0.050.10.150.20204060801000 Use this graph to answer the following questions: What is the half life of the reaction? Round your answer to significant digits. Suppose the rate of the reaction is known to be first order in . Calculate the value of the rate constant . Round your answer to significant digits. Also be sure you include the correct unit symbol. Predict the concentration of in the engineer's reaction vessel after seconds have passed. Assume no other reaction is important, and continue to assume the rate is first order in . Round your answer to significant digits.arrow_forwardThere is heavier traffic in the L.A. basin in the mornings and in the evenings as workers go to and from work in downtown L.A. Consequently, the flow of CO into the L.A. basin might be better repre- sented by the sine function over a 24-hour period. P1-5, The reaction A → B is to be carried out isothermally in a continuous-flow reactor. The entering volumetric flow rate vo is 10 dm/h. (Note: FA = C,v. For a constant volumetric flow rate v=v,, then FA = C,vo. Also, CAo = FA/vo = ([5 mol/h\/[10 dm³/h]) 0.5 mol/dm³.) Calculate both the CSTR and PFR reactor volumes necessary to consume 99% of A (i.e., CA = 0.01CA0) when the entering molar flow rate is 5 mol/h, assuming the reaction rate –ra is mol (a) -ra = k with k = 0.05 [Ans.: VesTR = 99 dm³] h-dm3 (b) -ra = kCA with k = 0.0001 s-! dm3 (c) -ra = kC with k = 300 mol ·h [Ans.: VesTr = 660 dm³] (d) Repeat (a), (b), and/or (c) to calculate the time necessary to consume 99.9% of species A in a 1000 dm³ constant-volume batch reactor with CA0…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The