
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
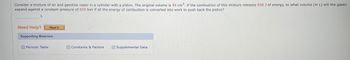
Transcribed Image Text:Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 45 cm³. If the combustion of this mixture releases 936 J of energy, to what volume (in L) will the gases
expand against a constant pressure of 659 torr if all the energy of combustion is converted into work to push back the piston?
L
Need Help? Read It
Supporting Materials
Periodic Table
Constants & Factors
Supplemental Data
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why do people use Calorimetry and discuss it's applications in the medical or engineering world.arrow_forwardwhat is the final volume of a balloon in which the change in internal energy increased by 50.0J, the heat increased by 70J, the initial volume is 0.50L, and the pressure of the balloon is 1 atm? Has the balloon done work or has work been on the balloon?arrow_forwardIf a balloon has an initial volume of 0.0 L, and inflating the balloon against an atmospheric pressure of 0.95 atm requires -250 J of work, what volume is the balloon once inflated?arrow_forward
- What is the change in internal energy of the air within the piston? The air within a piston equipped with a cylinder absorbs 555 J of heat and expands from an initial volume of 0.10 L to a final volume of 0.87 Lagainst an external pressure of 1.0 atm.arrow_forwardA gas expands and its pressure and volume are recorded, as shown above. What is the work done by the gas? (see picture attached) A. 130 J B. -13,172 J C. 13,172 J D. -130 Jarrow_forwardHistory Bookmarks Window Help Sun Mar 27 10:53 PM cvg.cengagenow.com 中 [References] Use the References to access important values if needed for this question. Ignition wires heat Thermometer Stirrer A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of combustion of sample fuels and the energy content of foods. In an experiment, a 0.5650 g sample of quinizarin (C14H804) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.187×103 g of water. During the combustion the temperature increases from 23.98 to Water 26.55 °C. The heat capacity of water is 4.184 J gloC1. The heat capacity of the calorimeter was determined in a previous experiment to be 770.8 J/°C. Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of quinizarin based on these data. C14H804(s) + 1402(g) → 4 H20(1) + 14 CO2(g) + Energy Sample dish Burning sample Steel bomb Insulated outside chamber Molar Heat of…arrow_forward
- A typical bike tire has a volume of 150 in³. If a bicycle pump can hold 567 cm³ of air, and you compress it to 350 cm³ with each pump, against an external pressure of 1.0 atm, how much total work (J) would you need to do on the gas in the bicycle pump in order to completely fill the tire? Give your answer as a positive value. Type your numeric answer and submitarrow_forwardthermor A 59.1 g sample of brass, which has a specific heat capacity of 0.375 J-g that contains 100.0 g of water. The temperature of the water starts off at 17.0 °C. When the temperature of the water stops , is put into a calorimeter (see sketch at right) insulate containe water changing it's 20.5 °C. The pressure remains constant at 1 atm. sample - Calculate the initial temperature of the brass sample. Be sure your answer is rounded to 2 significant digits. a ca Check Explanation 2021 McGraw H LLC A Rights eserved Address pe here to searcharrow_forwardThe question is given like this, it is not incomplete. There is no heat capacity of the calorimeter given. Can it be solved without that? It is high school chemistry so maybe it can be solved in an easier way? Many thanks.arrow_forward
- A sample of gass occupies a volume of 56.4mL. As it expands, it does 133.3J of work on its surroundings at a constant pressure of 783 torr. What is the final volume of gas?arrow_forwardQ2: An ideal gas is compressed from 6.0 L to 2.0 L by a constant external pressure of 4.0 atm. How much work (in KJ) is done on the gas? Q3: A 20.0 mL sample of a gas is at 7.00 atm of pressure and a temperature of 289 K. What volume (ml) would the gas occupy at STP?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY