
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Based on the formula for pressure-volume work, if work is done on the system by the surroundings, is the final volume larger or smaller than the initial volume?
Expert Solution
arrow_forward
Step 1
Given information,
Work is done on the system by the surroundings.
The pressure-volume formula is given by,
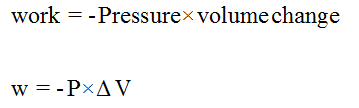
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the quantity of work required if He gas expanded from 1 L to 2 L by constant pressure of 2 atm.arrow_forwardA gas is compressed in a cylinder from a volume of 30.0 L to 18.0 L by a constant pressure of 1.32 atm. Calculate the amount of work done on the system.arrow_forwardA sample of gas occupies a volume of 52.5 mL. As it expands, it does 139.8 J of work on its surroundings at a constant pressure of 783 Torr. What is the final volume of the gas?arrow_forward
- During a breath, the average human lung expands by about .50 L. If this expansion occurs against an external pressure of 1.0 atm, how much work (in joules) is done during expansion?arrow_forwardI can determine mathematically how much energy will be produced or absorbed for a given chemical reaction… using Hess’ law for a net reaction from a series of reactions Are you able to provide an example of the question asked with step by step work ?arrow_forwardA chemical reaction requires 369.42 kJ of heat and performs 73.7 kJ of work on the surroundings. What is the exchange of internal energy in the reaction?arrow_forward
- A sample of helium behaves as an ideal gas as it is heated at constant pressure from 273 K to 356 K. If 21.0 J of work is done by the gas during this process, what is the mass of helium present? mgarrow_forwardmake sure that the answer is in the correct number of significant digitsarrow_forwardCalculate the work done when a gas at a pressure of 2.4 atm and constant temperature expand in volume from 1.0 to 2.2L.arrow_forward
- An automobile engine provides 585 Joules of work to push the pistons. In this process the internal energy changes by -2759 Joules. Calculate q for the engine. This represents the amount of heat that must be carried away by the cooling system.arrow_forwardA sample of gass occupies a volume of 56.4mL. As it expands, it does 133.3J of work on its surroundings at a constant pressure of 783 torr. What is the final volume of gas?arrow_forwardA sample of an ideal gas at 24.9 atm and 15.4 L is allowed to expand against a constant external pressure of 3.93 atm at a constant temperature. Calculate the work in units of kJ for the gas expansion. (Hint: Boyle’s law applies.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY