
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Work question out step by step. Include all steps, equations, and ALL answers and values!
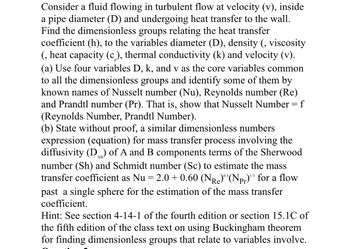
Transcribed Image Text:Consider a fluid flowing in turbulent flow at velocity (v), inside
a pipe diameter (D) and undergoing heat transfer to the wall.
Find the dimensionless groups relating the heat transfer
coefficient (h), to the variables diameter (D), density (, viscosity
(, heat capacity (c), thermal conductivity (k) and velocity (v).
(a) Use four variables D, k, and v as the core variables common
to all the dimensionless groups and identify some of them by
known names of Nusselt number (Nu), Reynolds number (Re)
and Prandtl number (Pr). That is, show that Nusselt Number = f
(Reynolds Number, Prandtl Number).
(b) State without proof, a similar dimensionless numbers
expression (equation) for mass transfer process involving the
diffusivity (D) of A and B components terms of the Sherwood
number (Sh) and Schmidt number (Sc) to estimate the mass
transfer coefficient as Nu 2.0+ 0.60 (NR)°³ (Npr)" for a flow
past a single sphere for the estimation of the mass transfer
coefficient.
=
Re
0.5
Hint: See section 4-14-1 of the fourth edition or section 15.1C of
the fifth edition of the class text on using Buckingham theorem
for finding dimensionless groups that relate to variables involve.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Write a report on Reynolds Number's experience. Where the report includes: 1- An introduction to the experience. 2- Steps of the experiment. 3. The purpose of the experiment. 4- The device used in the experimentarrow_forwardGive an example of an AE unit for energyarrow_forwardHow about the other parts? Can you summarize the final answers by listing them down at beginning?arrow_forward
- How should your lab notebook look like including title, purpose, and procedure?arrow_forwardQ2/A) Reduce the following below using Masson's method: H9 G3 -H3 -H₁ H₂ H7 -H8 G10 G9 G5 G6 G H4 -H5 H6.arrow_forwardA cube of metal is 1.32 cm on an edge. It has a mass of 21.3 g. Calculate the density of this metal cube. Show work for each step including the units in each step.arrow_forward
- What is a residue-curve map?arrow_forwardPrepare a table in which the rows are: length, area, volume, mass, and time. Make two columns, one for the SI and the other one for the English systems of units. Fill in each row with the name of the unit, and in the third column, show the numerical equivalency, i.e., 1 ft = 0.3048 marrow_forwardQ4/ Reduce the following system by using Masson's method: G7 H8 G5 G6 G4 G₁ G2 G3 H4 H5 H6 H₁ H₂ H3 G8 H7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The