
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please help me answer all with diagram, asap. Thank you!
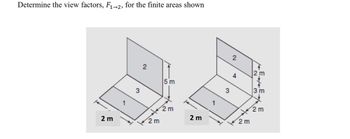
Transcribed Image Text:Determine the view factors, F₁-2, for the finite areas shown
2 m
3
2
2 m
←
5 m
2 m
2 m
3
2
A
2 m
~w N
2 m
3 m
2 m

Transcribed Image Text:View factor F12
0.50
0.40
0.30
0.20
0.10
Y= 0.1-
1.0
0.2
0.3
0.4
0.6
0.8
1.0
1.5
3.0
8.0
USE THIS FIGURE FOR NO.2
Dimension ratio, Y = 0.1
2.0
2.0
4.0
Scale changes here
3.0
4.0
Dimension ratio, Z
6.0
A₂
Y=y/x
Z=z/x
A₁
A₁ = Area on which heat-
transfer equation is based
Asymptotes
6 8 10
1
I
Figure 23.13 View factor for adjacent rectangles in perpendicular planes.
(From H. C. Hottel, “Radiant Heat Transmission,” Mech. Engrg., 52 (1930). By permission of
the publishers.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- AutoSave Document1 - Word O Search (Alt+Q) ff RICAMAE SALADAGA RS File Home Insert Draw Design Layout References Mailings Review View Help P Comments B Share X Cut O Find v Calibri (Body) - A A Aa v A AаBЬСcDd AaBbСcDd AaBbC AаBЬСc[ Аа B АавьссD v 11 LE Copy Replace Paste BIUV ab x, x A . I v A - I Normal 1 No Spac. Heading 1 Heading 2 Title Subtitle Dictate Sensitivity Editor Reuse S Format Painter A Select v Files Clipboard Font Paragraph Styles Editing Voice Sensitivity Editor Reuse Files |· 2: 1 1 |· ·1: 3.1. 4 5.. 6 . I 7.1· 8.I 10.1 11.| 12:1 13. 14. I 15. 16 17.1 18 | 19 II. The adiabatic exothermic irreversible gas-phase reaction 2A + B → 20 is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in the figure: 500,000 400,000 300,000 FAO -rA 200,000 100,000 0.3 0.6 0.9 1. What PFR volume is necessary to achieve 50% conversion? 2. What CSTR volume is necessary to achieve 50% conversion? 3. What is the volume of a…arrow_forwardWhat is a residue-curve map?arrow_forwardStuck need help! Problem is attached. please view attachment before answering. Really struggling with this concept. Please show all work so I can better understand ! Thank you so much.arrow_forward
- Hello how about #8?arrow_forwardIn a process with recycle and purge, which quantity stays the same between the recycle and purge stream at the recycle/purge splitting point ?arrow_forwardCold working is done at temperatures O A. below the room temperature O B. below the recrystallization temperature O C. below ductile-to-brittle transition temperature O D.below the melting point A Moving to another question will save this response. AL O Éarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The