
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
2a and 2b
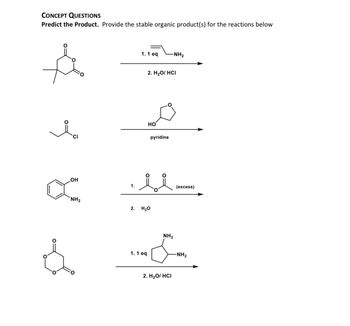
Transcribed Image Text:**Concept Questions**
**Predict the Product.** Provide the stable organic product(s) for the reactions below.
1. The top reaction:
- **Reactants:**
- Left: Cyclohexane-1,3-dione with a methyl group at one of the dione carbons.
- Right: Propylamine (1 equivalent).
- **Steps:**
- 1. 1 eq. Propylamine (NH₂)
- 2. H₂O / HCl
2. The second reaction:
- **Reactants:**
- Left: Acetyl chloride (ethanoyl chloride).
- Right: Tetrahydrofuran-2-ol.
- **Steps:**
- 1. Pyridine
3. The third reaction:
- **Reactants:**
- Left: 2-aminophenol.
- Right: Diketene (excess).
- **Steps:**
- 1. Diketene (excess)
- 2. H₂O
4. The bottom reaction:
- **Reactants:**
- Left: 1,3-benzodioxole-4,5-dione.
- Right: Piperazine (1 equivalent).
- **Steps:**
- 1. 1 eq. Piperazine (NH₂)
- 2. H₂O / HCl
---
In each of the above reactions, the stable organic products formed will depend on the interaction between the reagents and their functional groups, along with the reaction conditions specified.
- The first reaction typically results in the formation of an imine due to the reaction of a ketone and amine, followed by hydrolysis in acidic conditions.
- The second reaction is an esterification reaction facilitated by pyridine which may result in the formation of an ester.
- The third reaction involves the formation of a lactam when diketene reacts with 2-aminophenol and water hydrolyzes it.
- The fourth reaction may produce a hydroxamic acid through reaction with piperazine followed by hydrolysis.
Detailed mechanisms and exact product structures would require drawing tools and a step-by-step mechanistic description.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi! I am unsure of how to solve this problem. Thank you!arrow_forward18) What will the temperature of a 2100. mL sample of nitrogen at 526. mm Hg and 42.4oC be, in oC, after it changes to 1290. mm Hg and 3100. mL? Put your answer with 3 significant digits.arrow_forwardGiven the Volumetric Analysis of a Natural Gas: CH4 = 67.3% Nitrogen = 9.6% O2 = 2.7% CO = 0.8% C2H6 = 15.4% CO2 = 1.8% H2 = 2.4% Calculate the A/F ratio by mass considering CO = 6.1% CO2. Note: Use four (4) decimal places in your solution and answer.arrow_forward
- The U.S. proven natural gas reserves in 2013 were 323 trillion ft3 . How long will these reserves last if there are no imports or exports and if the U.S. annual rate of use of 24.5 trillion ft3 continues?arrow_forwardA commercial 737 jet transporting 143 passengers and 5 crew members from Kansas city (MCI) to Baltimore (BWI) burned 11800 lb. of Jet A fuel. Jet A fuel is kerosene based, consisting primarily of CnH2n+2 hydrocarbons, with n = 6 to 16, so the Carbon:Hydrogen ratio is close to 1:2. During this flight , how much CO2 (in kg) was released into the atmosphere? (assume the combustion of fuel was complete, so all fuel was converted into H2O and CO2).arrow_forwardWhich of the following equations is correct? A. PV = F + GB. F – G = PVC. F = G – PVD. G + F = 2TSarrow_forward
- Standard temperature and pressure are (1atm=760mmHg)arrow_forwardEthylene (CH₂CH₂) is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about 8.0 × 100 kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane (CH3CH3) from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas. Suppose an engineer studying ethane cracking fills a 50.0 L reaction tank with 20.0 atm of ethane gas and raises the temperature to 600. °C. She believes K = 0.70 at this temperature. р alo Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. Round your answer to 2 significant digits. Ar Note for advanced students: the engineer may be mistaken about the correct value of K, and the mass percent of ethylene you calculate may not be what she actually observes. %…arrow_forwardSince the specific volume of nitrous oxide gas (N 2 O) at a pressure of 100 kPa and a temperature of 47 ° C multiplied by the compressibility factor is 0.453 m ^ 3 / kg, what is the compressibility factor of this gas?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY