
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
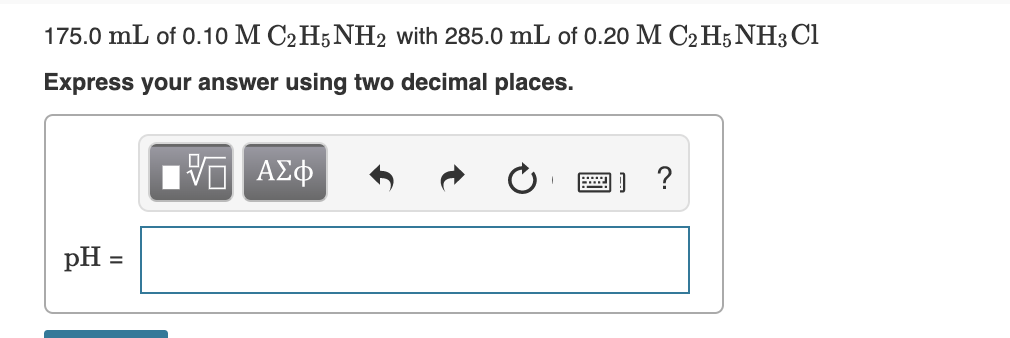
Transcribed Image Text:175.0 mL of 0.10 M C2H5NH2 with 285.0 mL of 0.20 M C2 H5NH3 Cl
Express your answer using two decimal places.
ΑΣφ
......
pH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- x P UTF-8"Chapter 12 Solutions -20 X 2-xythos.content.blackboardcdn.com/5b19550bob538/10621560?X-Blackboard-Expiration%=1649052000000&X Vorksheet.docx Edits will not be automatically saved. Save now d changes A Garamond 14. 三 三 of NaHCO3 6. What is the molarity of 325 ml of a solution containing 46.8 (Molar 84.007 g/mol) | mass =arrow_forwardQuestion 25 of 30 Submit 300.0 mL of a 0.300 M solution of Nal is diluted to 700.0 mL. What is the new concentration of the solution? | M 1 4 6. C 7 +/- x 10 0 Tap here or pull up for additional resources 3. LO 00arrow_forward22:48 Tue May 4 Done < AA A session.masteringchemistry.comarrow_forward
- 100mL of 0.5 M sodium hydroxide was used to completely titrate 150 mL of H3PO4. What was the original molar concentration of the H3PO4 solution?arrow_forward- 3 At 25.0 °C the Henry's Law constant for phosphine (PH,) gas in water is 8.1 x 10 °M/atm. Calculate the mass in grams of PH, gas that can be dissolved in 850. mL of water at 25.0 °C and a PH, partial pressure of 2.57 atm. Round your answer to 2 significant digits.arrow_forwardProblem 10.51 - Enhanced - with Feedback 46 of 50 I Review | Constants | Periodic Table Part A What is the molarity of an HCl solution if 6.00 mL HCl solution is titrated with 20.6 mL of 0.175 M NaOH solution? HCl(aq) + NaOH(aq)→NaCl(aq) + H2O(1) Express your answer numerically in molarity. ΑΣΦ ? molarity = M Submit Request Answer Provide Feedback Next >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY