
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
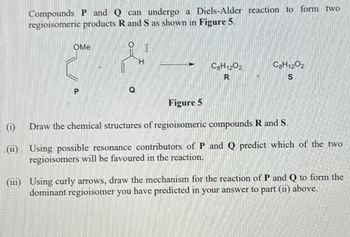
Transcribed Image Text:Compounds P and Q can undergo a Diels-Alder reaction to form two
regioisomeric products R and S as shown in Figure 5.
OMe
P
H
C8H1202
R
C8H1202
S
Figure 5
Draw the chemical structures of regioisomeric compounds R and S.
(ii) Using possible resonance contributors of P and Q predict which of the two
regioisomers will be favoured in the reaction.
(iii) Using curly arrows, draw the mechanism for the reaction of P and Q to form the
dominant regioisomer you have predicted in your answer to part (ii) above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the diene and dienophile expected to produce the Diels-Alder product shown in the box below. SO 11 SO IV ον O. IV 4 ||| ||| ||arrow_forward1) Azulene, bicyclo[5.3.0] decapentaene, is an beautiful blue colored hydrocarbon with a large dipole moment (for a hydrocarbon). Draw resonance structures that could explain the large dipole moment. Azulenearrow_forward10.36 A very large number of Diels-Alder reactions are recorded in the chemical literature, man O your knowl of which involve relatively complicated dienes, dienophiles, or both. On the basis of edge of Diels-Alder reactions, predict the constitution of the Diels-Alder adduct that you would expect to be formed from the following combinations of dienes and dienophiles: Also (b) 7 O + CH₂O₂CC=CCO₂CH, -carbocation/radical structure, stability and resonancearrow_forward
- Which of the following can act as the diene in a Diels-Alder reaction? Choose all that apply. 0 CH3O none of these applyarrow_forwardPlease don't provide handwriting solutionarrow_forwardIs the following true or false? To catalyse a Diels-Alder reaction a common approach involves the use of a Lewis acid capable of lowering the LUMO of the dienophile.arrow_forward
- Draw the structure of the product of the Diels-Alder reaction below. H3C CH3 O || O || + CH3OC-CEC-COCH3arrow_forward4. :Ö N-Phenylsydnone, so-named because it was first studied at the University of Sydney, Australia, behaves like a typical aromatic molecule. Explain, using the Hückel 411 + 2 rule. H N 2-2 Z-1 :0-N N N-Phenylsydnone :0: :0 :0 H N 2: N- 2=arrow_forwardDraw the major product of the following Diels-Alder reaction. Use the Bicyclic ring tool and guide points to draw bicyclic molecules. NC CN A Click and drag to start drawing a structure. X Ö 4 Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY