
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Compound A reacts with 1 molar equivalent(s) of hydrogen upon catalytic hydrogenation. A undergoes reaction with ozone, followed by Zn treatment, to give:
Propose a structure for A.
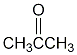
Transcribed Image Text:The image depicts the structural formula of acetone (propanone). It consists of three carbon atoms, six hydrogen atoms, and one oxygen atom arranged as follows:
- A central carbon atom is double-bonded to an oxygen atom, forming a carbonyl group (C=O).
- This carbon is also singly bonded to two CH₃ groups (methyl groups), one on each side.
The diagram visually represents the molecular structure of acetone, illustrating its functional group and connectivity of atoms. Acetone is a simple ketone widely used as a solvent and in the production of plastics and other industrial materials.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Both pyrrole and pyridine are nitrogen containing heterocycles that are 6π Hückel aromatic. Describe the aromaticity and observed basicity for that molecule, which is more basic, explain and show the acid -base reaction involved.arrow_forwardAnswer ALL parts. (a) Treatment of enone C with tetrabutylammonium fluoride leads to the formation of heterocycle D. 2 steps BU4NF C9H1602 A + B D OSIME,tBu (i) Enone C can be prepared from alkyne A and aldehyde B in two steps via a gold-catalysed reaction. Identify A and B and provide reagents/conditions for a synthesis of C. (ii) Give the structure of D and classify the ring-closing reaction for its formation from C according to Baldwin's rules (iii) Sketch the key molecular orbitals involved in the ring-closing reaction in part (ii) and hence explain whether the reaction is favourable or unfavourable.arrow_forwardGive detailed Solution with explanationarrow_forward
- Sinthesis of 2,3-diphenylquinoxaline: which produce as a by product? HCl NH3 H2 H2Oarrow_forwardPropose a reasonable synthesis for each of the following compounds from the indicated starting materials and any other reagents.arrow_forward3. What starting material would be necessary to prepare the following compound at on by the Diels-Alder reaction? RMA aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY