
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Suggest the reagents for the synthesis of the following compounds
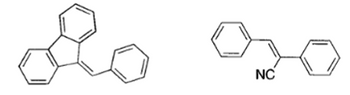
Transcribed Image Text:NC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ph3P-CHCH3 H₂C H3C Aldehydes and ketones are converted into alkenes by means of a direct nucleophilic addition called the Wittig reaction. In the reaction, a triphenylphosphorine ylide, also called a phosphorane, adds to an aldehyde/ketone to give a four-membered cyclic intermediate called an oxaphosphetane. The oxaphosphetane is not isolated but instead spontaneously decomposes to release triphenylphosphine oxide and an alkene. CH3 00 + The ylide is formed by reaction of triphenylphosphine, a good nucleophile, with a primary alkyl halide in an SN2 reaction, followed by deprotonation of the carbon with a strong base, such as butyllithium. The carbonyl carbon and the carbon originally bonded to the halogen become the two carbons with the double bond in the product alkene X m CH3 The real value of the Wittig reaction lies in its ability to yield an alkene of predictable structure, as the C=C bond is precisely where the C=O bond was in the reactant and no isomers (other than E/Z isomers)…arrow_forwardthe organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forward47) Provide the structure of the major organic product which results in the following reaction. Br KI Br CH3arrow_forward
- how to synthesize 2-phenylclohexanone from cyclohexanone?arrow_forwardWhich of the following reactions will synthesize phenol from benzene? 1) HNO3 + H2SO4; 2) Fe, HCl; 3) NaNO2, HCl, 0-10 oC; 4) warm H2SO4 and H2O 1) HNO3 + H2SO4; 2) Fe, HCl; 3) NaNO2, HCl, 0-10 oC; 4) CuCN; 5) dilute acid and heat 1) Acetyl chloride & AlCl3; 2) bleach 1) Ph-N2+ + KI; 2) BrMgCH=CH2 in ether, followed by H3O+; 3) warm, conc'd KMnO4 1) Cl-CH(CH3)-CH2CH2CH3 + FeBr3; 2) hot, conc'd KMnO4arrow_forward27) Give the products of the reaction and provide a step-by-step mechanism for the formation of the products. O not + H₂O HCIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY