
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
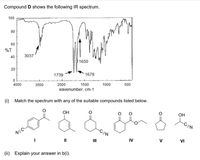
Transcribed Image Text:Compound D shows the following IR spectrum.
100
80
60
%T
40
3037
1650
1739
1678
4000
3000
2000
1500
1000
500
wavenumber, cm-1
(i) Match the spectrum with any of the suitable compounds listed below.
ОН
OH
-CEN
-CEN
NEC-
II
II
IV
V
VI
(ii)
Explain your answer in b(i).
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A molecule produces two molecular ions with a m/z of 152 and 154 and a base peak with a m/z of 73. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 1H 11 10 3000 9 8 2000 7 1500 Wavenumbers (cm) + 6 5 2H 1000 2H 2 1 500 ppm of Q Qarrow_forward18. Proton NMR spectra are given together with molecular formula. Propose a structure that corresponds to each spectrum. (Hint: Find IHD first). Assign peaks to show which protons give rise to which peaks 10 10 10 (b) C4H8O₂ Offset: 2.4 ppm (b) C₂H100 9 (c) CsHgO₂ 9 8 8 7 7 6 6 6 5 8 (ppm) OHz 5 8 (ppm) 4 3.08 2.98 2.88 2.78 8 (ppm) 50Hz 4 4 3 2 1 0 0arrow_forwardPlease clearly label the answer and explain thoroughly. Thanks in advance.arrow_forward
- Please list all major chemical Shifts, Integration, Splitting, and Conclusion for the 1H-NMR peaks.arrow_forwardGive detailed Solution with explanation needed...don't give Handwritten answer. don't copy answer anywhere. Don't use Ai for answering thisarrow_forwardWhich one of the given compounds is consistent with the IR spectrum shown? 100 Transmittance T OH 300 Courtesy of SDBS: National Institute of Advanced Industrial Science and Technology OH wavenumber am IV M 1000 H 300 Harrow_forward
- Analyze the CNMR spectrum below and identify the compoundarrow_forwardInterpet and analyze this Infrared (IR) spectra to show the signs from the peaks and what they tell about the unknown compound.arrow_forwardPropose a structure that is consistent with the following 1H NMR spectra. In each case, the molecular formula is provided. For spectra with numbers at the bottom of the signal you will need to determine the integration, others with numbers above the signal integration has been determined for you.arrow_forward
- Provide a reasonable structure for each IR spectrum with the indicated molecular formula. Show how you arrive at each structurearrow_forwardDraw the structure of the acid chloride with a molecular formula of C8H7ClO and the following spectroscopic data:arrow_forwardWhat structure is consistent with these spectra? PPM 100 Кey Peaks m/z = 27, 43, 107, 50 109, 150, 152 50 100 150 m/z al Br-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY