
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
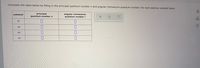
Transcribed Image Text:Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed.
principal
quantum number n
subshell
angular momentum
quantum number /
dlo
5f
2p
6d
3s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Is this set of quantum numbers possible? n = 2, l = 1, ml = 0, ms = +1/2arrow_forwardFor each of the following sets of quantum numbers, determine how many electrons in a single atom could have that specific set of numbers. n = 2 Number: n = 4, l=1 Number: n = 5,l=1, m₁ = 0 Number: electrons electrons electronsarrow_forwardTwo electrons can have the same quantum numbers n=3, l=1, ml=-1. True or Falsearrow_forward
- The electron configuration of an atom tells us how many electrons are in each orbital. For example, helium has two electrons in the 1s orbital. Therefore the electron configuration of He is 1s^2. What is the ground-state electron configuration of a neutral atom of cobalt? Express your answer in condensed form, in order of increasing orbital energy. For example, [He]2s^22p^2 would be entered as He][He]2s^22p^2 .arrow_forwardComplete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. subshell 7p 3d 5s 2s principal quantum number n 1 1 angular momentum quantum number / X Śarrow_forwardHow many electrons in the quantum number of the atom n = 5, l = 2, ml = -1arrow_forward
- Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. principal quantum number n angular momentum quantum number / subshell 6d 5f 7s 2parrow_forwardHow many electrons in an atom can have the following sets of quantum numbers? Enter the maximum number of electrons into the table. Quantum Numbers n = 2, 1 = 0 n = 4,1 = 3 n = 2 n = 2, 1 = 0, m₁ 0, ms = -1/2 Number of Electronsarrow_forwardWhat is the I quantum number for an electron in 8 d?arrow_forward
- how many electrons in an atom of Ar have the quantum numbers: n=3, l=2, m=0? 6 0 10arrow_forwardComplete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. principal quantum number n subshell angular momentum quantum number / 5f 6s 4p Зр ロ□□□arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY