
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
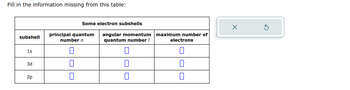
Transcribed Image Text:Fill in the information missing from this table:
subshell
1s
3d
2p
Some electron subshells
principal quantum
number n
0
П
0
angular momentum
quantum number /
U
maximum number of
electrons
0
П
0
X
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the physical explanation of the difference between a particle having the 3-D rotational wavefunction 3,2 and an identical particle having the wavefunction 3,2?arrow_forwardDraw, label, and explain the functions of the parts of a spectroscope.arrow_forwardPhotoelectron spectroscopy is s1milar to the photoelectric effect (Section 6.2). However, in the photoelectric effect, electrons are ejected when light strikes the surface of a(n)arrow_forward
- what is the absolute energy (wavenumbers) for hydrogn from n=6 to n=1arrow_forwardHi I need help on one of my physical chemistry questions and I don't know how to do it. Will you please help me solve for it thank you very much!arrow_forwardPlease answer both questions!! Please make sure it's handwritten and legible!!!arrow_forward
- Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number? A 2d 2s All of these D None of these E 2parrow_forward5. (McQuarrie 9-25) Show that the atomic determinantal wave function, 1 1sa (1) 1sẞ(1) V(r1, r2)= √2 1sa (2) 1sẞ(2) is normalized when the 1s orbitals are normalized. Hint: Don't freak out about this problem-it's easier than it looks! Expand the Slater determinant to get the wavefunction in a more usable form, then set up the normalization integral for . Multiply everything out, and you should have complicated-looking integrals such as this: (1sa (1) 1sẞ(2) | 1sa(1) 1sẞ(2)) While such integrals might look scary, they're actually straightforward. An integral like the one above has four pieces: integration over (a) the spatial coordinates of electron 1, (b) the spatial coordinates of electron 2, (c) the spin coordinates of electron 1, and (d) the spin coordinates of electron 2. You can break up these multi-dimensional integrals into separate integrals for each piece. For example: (1sa (1) 1sẞ(2) | 1sa(1) 1sẞ(2) ) = (1s(1)|1s(1)) · (a(1)|a(1)) · (1s(2)|1s(2)) · (6(2)|ß(2)) Then you can…arrow_forwardA hydrogen atom is immersed in a magnetic field so that its energy levels split according to the Zeeman effect. Neglecting any effects due to electron spin, how many unique energy levels are available to an electron in the 5f subshell?arrow_forward
- 3. Verify that the wave function p(x) = Ae-[(ku)¹/² x²/(zh)] (where 4. m. and kare A, m, all constants) is a solution to the Schrodinger equation ħ² d² 1-2 u |- ²2μ dx² + ½kx²] p(x) = Eq(x), and find the energy in terms of m, and k. j 2 horva Guation BossMabayarrow_forwardThe wavefunction for the 2s orbital of a hydrogen atom is N((2-r)/a0)e-r/2a0. Evaluate the normalization constant N.arrow_forwardConstruct the wavefunction for an excited state of the He atom with configuration 1s13s1. Use Zeff = 2 for the 1s electron and Zeff = 1 for the 3s electron.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning