
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Only use the answers soluable or insoluble
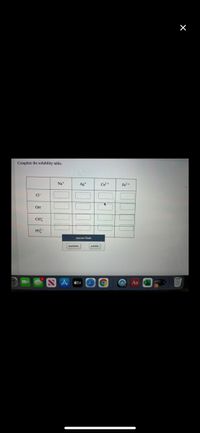
Transcribed Image Text:Complete the solubility table.
Na*
Ag+
Ca?+
Fe?+
CI
OH
CIO
PO
Answer Bank
insoluble
soluble
tv
Aa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume (mL) of of 0.421 M H₂SO solution is to be added to 22.3 mL of 0.419 M LIOH solution in the reaction below: H₂SO (aq) + 2 LIOH (aq) USO (aq) • CHO Do not put units in your answer. Report your answer to one decimal place.arrow_forwardIn a solution the substance which is being disolved is thearrow_forwardMatch the definition with it's correct word. A mixture composed of a solute dissolved in a solvent. The greater substance that does the dissolving in a solution. A term that describes when a solid solute does not dissolve in a solid solvent. [Choose ] [Choose ] Soluble Solvent Solution Insoluble Miscible Immiscible Solute >arrow_forward
- The correct definition of the Ksp is ..... a. The product of the number of particles of the solution raised to the coefficient Cb. The product of the mole of ions in solution raised to the coefficient c. Product of the concentration of the ions in the unsaturated solution d. The product of the number of ions in the solution raised to the coefficient Ce. The product of the concentration of the saturated solution ions is raised to the coarrow_forwardwhat is a soluble reactivity shim?arrow_forwardcacoH)2 a 15L he molarity of Our created Bi3 grams of is dissolved to create solution.calulate T solutionarrow_forward
- You are a budding inventor who has just manufactured their own toy rocket from scratch. As your dream is to manufacture the most realistic toy ever, you wish to create even the rocket fuel. You don’t know how long you will need to store the fuel components for, so you will leave the Ammonium perchlorate in solution form until required. This ammonium perchlorate solution is made by combining 100 mL 1.2 mol Perchloric acid (HClO4) and 100 mL 2.0 mol of Ammonia (NH3). You need to know whether this solution is acidic or basic to ensure you store it safely and properly. What is the pH of the solution you created? Full solution pleasearrow_forwardHow many grams of CrF, will dissolve in 250 mL of water? For CrF, the molar mass is 108.99 g/mol and Ken = 6.6 x 1011,arrow_forwardSome compounds are soluble in water and some compounds are insoluble in water. For each compound below determine if the compound if soluble or insoluble in water. * Soluble Insoluble Mg(NO:): Agcl LIOH Mg:(PO.):arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY