
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
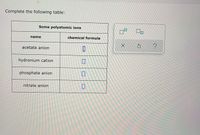
Transcribed Image Text:Complete the following table:
Some polyatomic ions
name
chemical formula
acetate anion
hydronium cation
phosphate anion
nitrate anion
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the missing numbers and charges. name formula dihydrogen phosphate H PO4 sulfite SO periodate IO chorate ClOarrow_forwardA general chemistry student was asked to write the chemical formula of the following compounds. The student's answers are given below. How many formulas are correct? OOOO 4 3 5 2 Compounds Aluminum perchlorate Cobalt(III) sulfite Hydrobromic acid Bromic acid Ammonium sulfate Student's proposed formula AI(CIO4)3 C0₂(SO3)3 HBrO(aq) HBrO3(aq) NH4SO4(aq)arrow_forwardDecide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided. empirical formula of ionic Forms ionic element #1 element #2 name of ionic compound compound? compound potassium barium O yes no cesium iodine yes no bromine potassium yes no sulfur chlorine yes noarrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation NaCl Co3(PO4)2 NH₂F FeBr₂ Fe(OH)3 Na 0 anion 0 X Sarrow_forwardComplete the table below by writing the symbols for the cation and anion that make up each ionic compound?.arrow_forwardDecide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided. empirical formula of ionic name of ionic compound compound Forms ionic element #1 element #2 compound? cesium bromine O yes O no sodium sulfur O yes O no strontium potassium O yes O no chlorine magnesium O yes O noarrow_forward
- Use elements and polyatomic ions listed in the box to create both chemical formulas and names. Use different compounds than the ones in rhe chart that is filled out.arrow_forwardWrite the formulas for the following ionic compounds. Please show the cation and polyatomic ion charges as part of your work Aluminum phosphate Iron (III) sulfate Calcium hydroxide Lead (IV) phosphate Tin (IV) oxide Iron (III) sulfidearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY