
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:D
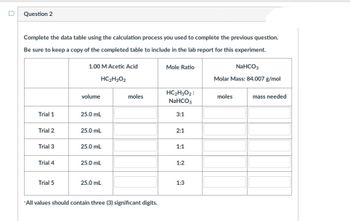
Question 2
Complete the data table using the calculation process you used to complete the previous question.
Be sure to keep a copy of the completed table to include in the lab report for this experiment.
Trial 1
Trial 2
Trial 3
Trial 4
Trial 5
1.00 M Acetic Acid
HC₂H3O2
volume
25.0 mL
25.0 mL
25.0 mL
25.0 mL
25.0 mL
moles
*All values should contain three (3) significant digits.
Mole Ratio
HC₂H3O2:
NaHCO3
3:1
2:1
1:1
1:2
1:3
NaHCO3
Molar Mass: 84.007 g/mol
moles
mass needed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Equal volumes of Solutions 2 and 3 were mixed in the lab. Suggest a measurement that could be made in the lab to provide evidence that a chemical reaction occurred when Solutions 2 and 3 were mixed.arrow_forwardWhat volume (in mL) of 30.0% hydrogen peroxide solution is required to provide the mass required in question 8 above? Mass=10.2g of a 30% hydrogen peroxide soltuion. Density of 30.0% H2O2 = 1.11 g/mL Answer with 3 sig figs.arrow_forwardYou may want to reference (Pages 306 - 308) Section 9.5 while completing this problem. Part A Determine the volume, in milliliters, required to prepare each of the following diluted solutions. 23.0 mL of a 0.250 M KNO3 solution from a 6.50 M KNO3 solution Express your answer with the appropriate units. HÁ Value Units Submit Request Answer Part B 28.0 mL of a 2.50 M H2SO4 solution from a(n) 12.4 M H2SO4 solution Express your answer with the appropriate units. HA ? Value Unitsarrow_forward
- Use sig figsarrow_forward3-6 3 SOLUTIONS AND NAME SOLUTION CONCENTRATIONS 1415 Section Pre-Lab Assignment Experiment objectives A, B, and C require you to devise procedures for preparing a saturated aqueous solution of potassium sulfate, experimentally determining the density of this solution, and experimentally determining the mass percent of potassium sulfate in this solution. Your pre-lab assignment is to develop procedures for parts A, B, and C. You will then execute these procedures in lab. PREPARATION OF A SATURATED AQUEOUS POTASSIUM SULFATE SOLUTION Reminder: use a maximum of 75 mL of water. A. В. DENSITY OF SATURATED AQUEOUS POTASSIUM SULFATE SOLUTION C. MASS PERCENT OF POTASSIUM SULFATE IN A SATURATED POTASSIUM SULFATE SOLUTIONarrow_forwardA solution consists of 35.00 g of CuSO, dissolved in 250.0 mL of water. The molar mass of Cu is 63.55 /mol, the molar mass of S is 32.07 /mol, and the molar mass of O is 16.00 /mol. What is the molarity of the solution? Show your work. PENCIL THIN BLACK 中arrow_forward
- If 10.0 mL of a 3.00 M H2SO4 stock solution are diluted with water to a final volume of 0.500 L, what will be the final concentration of H2SO4 in the dilute solution? Report your result in decimal notation and to the proper number of significant figures. All numbers are measured. a) 0.0300 Mb) 0.300 Mc) 0.150 Md) 0.0600 Me) 0.600 Marrow_forwardDetermine what volume (in mL) of 10.0 M HCl is equivalent to 0.800 mol of HCl. (Do not include the units in your answer.) Type your numeric answer and submitarrow_forwardb. Determine the number of moles of 0.01786 M acetic acid in 200 ml. (report 3 sig. fig.) liters x 1 moles liter 4. The demonstration used 1 g, 2g, 3g, 4g, and 5g of sodium bicarbonate, NaHCO3. a. Determine the molar mass of NaHCO3. Use 22.99, 1.01, 12.01, and 16.00 g mol-¹.arrow_forward
- Masses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forwardts/431885/variants/431885/take/15/ TEXT ANSWER Question 18 Calculate the molarity of a sodium hydroxide solution if 50.0 mL of this solution was completely neutralized by 46.6 mL of 0.750 M sulfuric acid. Balanced equation: H₂SO4+2NaOH → Na₂SO4 + 2H₂O Show your work. Use the following formula: X [NaOH] = 2x mL H₂SO4 x [H₂SO4] mL NaOH X₂ X² Normal AA 2 x 46.6= 93.2 1 Ďõõŏ õõ õõŏõõčõõčŏo O 20 of 20 Total Questions Answered 2 90 C N NJ H I III 123 B I US | √x ΩΡΑ Ix ((( All Changes Saved V ✰ ✰ 294 words left 7:56 P 5/2/202 ^ ☎ ☎ ‹ barrow_forwardCalculate the grams of solute needed to prepare each of the following. Part A You may want to reference (Pages 298 - 305) Section 9.4 while completing this problem. 2.00 L of a 2.00 M NaOH solution Express your answer with the appropriate units. HẢ ? Value Units Submit Request Answer Part B 4.80 L of a 0.195 M CaCl2 solution Express your answer with the appropriate units. HẢ ? Value Units Submit Request Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY