
a buffer solution is often encountered during the titration of a
weak acid. In such a titration, there is a strong base (often sodium hydroxide, as in today’s lab)
which is being added to the weak acid. When the strong base reacts with the weak acid, the
result is the conjugate base of the weak acid. It is essential that you not confuse these two
bases during the discussion below, and that you write your report so that it is clear which base
you are talking about. If the pH of the acid solution is monitored during the titration, a pH
profile like the one below can be plotted. For monoprotic acids it will be sigmoid in shape:
The Henderson-Hasselbalch equation helps to make sense of this curve (the base referred
to is the conjugate base of the weak acid).
pH = pKa + log ([base]/[acid])
If calculations are desired, two points are particularly important. The first, at the steepest point
of the graph, is the equivalence point. At that point the acid has been completely consumed by
the strong base being added, thus it can be used to stoichiometrically calculate the moles of
acid initially present.
The other important point is the half equivalence point. Notice in the Henderson-
Hasselbalch equation that the pH equals the pKa when the concentration of the weak acid and
its conjugate base are equal (since [base]/[acid] equals one, and the log of one equals zero).
The weak acid and its conjugate base are equal in concentration when exactly half of the weak
acid has been neutralized by the strong base. Since at the equivalence point the volume of
strong base was enough to react with all of the acid, the half equivalence point is at exactly half
of that volume of strong base. This point is important whenever a value for Ka is desired. titrating a solution of an unknown amino acid to
determine its molar mass and pKa value(s). With this information you will be able to identify
the specific amino acid. Amino acids are the fundamental building blocks of proteins, and thus
are of pivotal importance to biologists as well as chemists. They have the general structure
shown below; the typical amino acid has a hydrogen, a CO2– group, and an NH3+ group (and it is
one of these latter protons that is acidic). What differentiates one amino acid from the next is
the nature of fourth group, designated here with an R. This variable collection of atoms imparts
to each amino acid a different molar mass and chemical acidity; in the amino acids you will be
studying today, it will also possess a second acidic proton.
Question 1:
- Comment on the shape of your graph. Thoroughly explain the chemical cause of each flat or steep region of the graph using the image attached
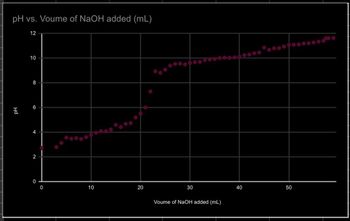
Step by stepSolved in 3 steps with 1 images

- What is the purpose of a titration? Explain the logic/steps of carrying out a titration, specifying the importance of each step in the process.arrow_forwardBe sure to answer all parts. For the titration of 30.0 mL of 0.150 M acetic acid with 0.100 M sodium hydroxide, determine the pH when: (a) 30.0 mL of base has been added. (b) 45.0 mL of base has been added. (c) 60.0 mL of base has been added.arrow_forwardA chemistry graduate student is given 450. mL of a 1.40M nitrous acid (HNO,) solution. Nitrous acid is a weak acid with K,=4.5 × 10 *. What mass of a NaNO, should the student dissolve in the HNO, solution to turn it into a buffer with pH = 3.61? 2. You may assume that the volume of the solution doesn't change when the NaNO, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. x10arrow_forward
- Please don't provide handwritten solution ....arrow_forwardA solution is prepared that is initially 0.18 M in hydrocyanic acid (HCN) and 0.44 M in sodium cyanide (NaCN). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in initial change final Explanation 80°F Mostly sunny [HCN] 0 0 0 Check x $ [CN] 0 0 0 с F5 % [H₂O*]. You can leave out the M symbol for molarity. [H₂O*] 0 0 F6 0 ✈ F7 믐 olo Q Search & X LOL F8 S * F9 C 12 DE ( F10 Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessib ☀- B. F11 ) *+ 7 F12 - PrtSc + 4 Insert U Delete Recke ►/11 Num 3arrow_forwardThe second buffer you will make in lab, Buffer B, will be made by combining the appropriate amounts of 1.8 M acetic acid and 1.0 M sodium hydroxide in a 100.00 mL volumetric flask. The concentration of the acetate, A–, in the buffer will be 0.32 M. Calculate the volume in milliliters (mL) of 1.0 M sodium hydroxide you need to add to get this concentration in your 100.00 mL solution.arrow_forward
- A student titrates a weak acid with 0.100M NaOH, adding 35.64 mL before a dark pink color is seen. Realizing that the end point has been passed, he then adds 2.77 mL 0.0500 M HCI until the solution is neutralized. How many moles of H* were present in the initial weak acid solution? Type answer:arrow_forwardCan someone explain to me why this example is a buffer? I don't understand it.arrow_forwardWhy is it important not to add too much HCl and go past the endpoint? O The volumne of acid added will be lower than expected for the calculations. O The color change can reverse. O The solution could bubble out of the flask. O The volume of acid added will reflect more than acid required to neutralize the base.arrow_forward
- Setting up a reaction table for a pH calculătióh With a COMMOR.. A solution is prepared that is initially 0.24M in ammonia (NH,), a weak base, and 0.28M in ammonium bromide (NH Br). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in OH . You can leave out the M symbol for molarity. он [NH,] [NH:] [on] do initial change final Explanation Check O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use| Privacy Accessibility 17 MacBook Air F10 ploarrow_forwardWhat is the pH of a buffer solution that is 0.493 M in hydrofluoric acid and is 0.483 M is potassium fluoride? The K, of hydrofluoric acid is 6.4 x 104. (Two decimal places) Type your answer...arrow_forward2. In the second titration, the molar mass of an unknown monoprotic acid is calculated. If 0.100 grams of unknown solid are dissolved in water and titrated with 4.68 mL of the NaOH, what is the molar mass of the solid? HINTS: Molar mass is grams / moles. We have grams from the weighed mass, we can calculate moles of acid from the titration. Monoprotic means that it produces 1 H+ per molecule. This means that our moles of NaOH added and moles of acid are the same. You will need to use your concentration of NaOH from the previous question Which is 0.1186arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





