
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How should I solve this? Could someone show the calculations for the varying mL concentrations? Also is there a difference between calculating an acid vs a base?
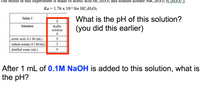
Transcribed Image Text:The buffer in this experiment is made of acetic acid HC2H3O2 and sodium acetate NaC2H3O2 (C2H;O2 ).
Ka = 1.76 x 10-5 for HC2H3O2
What is the pH of this solution?
(you did this earlier)
Table 1
Solution
Buffer
solution
A
acetic acid, 0.1 M (mL)
9
sodium acetate, 0.1 M (mL)
3
distilled water (mL)
After 1 mL of 0.1M NaOH is added to this solution, what is
the pH?

Transcribed Image Text:After 2 mL of 0.1M NaOH is added to this solution, what is
the pH?
After 3 mL of 0.1M NaOH is added to this solution, what is
the pH?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi! I have a homework question that states: a 3.48 M aqueous solution of NaCl is needed. In order to make 1500 ml of this solution, the mass of NaCl used is what? I have no idea where to even start on this one.arrow_forwardThe following diagrams are needed for questions 1 & 2. Water molecules are not shown for clarity. Each beaker contains the same volume of solution: Кey: = HA (unreacted acid) = A O = H* (or H30*) Beaker A Beaker B Beaker C Beaker D 0.1 mol/L HCI 1. Beaker A is representing a 0.1 mol/L HC1(aq) solution. Which acid might be the label on Beaker C? Why? A. 0.01 mol/L weak acid B. 0.1 mol/L weak acid C. 0.3 mol/L weak acid D. 0.01 mol/L strong acid E. 0.3 mol/L strong acidarrow_forwardQuestions 1-4 refer to the same strong acid/strong base (SA/SB) titration. A 25.00 mL solution of 0.200 M hydroiodic acid (HI) is being titrated with 0.200 M sodium hydroxide (NaOH). What is the solution pH when 30.00 mL of titrant have been added? (Two decimal places) Type your answer...arrow_forward
- can you help me and line it exactly like this because i keep doing it and its says its wrongarrow_forwardHow can we find the volume for both questions?arrow_forwardPlease help me answer questions 1 and 2. (Questions attached below) 1. When you arrive at the lab, 2.0 M H2SO4(aq) and 2.0 M HC2H3O2(aq) will be present. Youwill need to dilute these solutions using beakers and graduated cylinders from your drawerand DI water.i. Calculate the volume of the 2.0 M HC2H3O2(aq) and DI water needed to create 50.0 mLof 1.05 M HC2H3O2(aq).ii. Calculate the volume of the 2.0 M H2SO4(aq) and DI water needed to create 50.0 mL of0.75 M H2SO4(aq). 2. A 48.33-mL sample of 0.150 M NaBr is titrated into 50.00 mL of 0.145 M AgNO3. Bothsolutions had the same initial and final temperatures. The reaction occurred in a calorimeterwith a known heat capacity and produced 1546.03 J of heat.i. Write a balanced equation that is occurring.ii. Determine the theoretical yield of the solid.iii. Calculate the enthalpy of the reaction (kJ/mole of precipitate).arrow_forward
- Helparrow_forwardUse the formula pH=-log[H^+]. The variable pH represents the level of acidity or alkalinity of a liquid on the pH scale, and [H^+] is the concentration of hydrogen ions in the solution. Determine the value of [H^+] (in mol/L) for the following liquids, given their pH values. Express your answers in scientific notation with three significant digits. a.) Tea pH=5.5 b.) Milk pH=6.2 What is the concentration of hydrogen ions in tea? What is the concentration of hydrogen ions in milk?arrow_forwardIf 50.1 mL of 0.128 M hydrochloric acid requires 17.5 mL of NaOH solution to reach the endpoint, what is the molarity of the NaOH solution? Include three decimal places in your answer.arrow_forward
- (use SF, units; show all work; answers in box) 1. a: Acetic acid (HC2H302) is a weak electrolyte. What substances are present in HC2H3O2 (ag)? b: Chloric acid (HCIO3) is a strong electrolyte. What substances are present in HCIO3 (aq)? 2. Identify each of the following substances as a strong electrolyte, weak electrolyte, or nonelectrolyte: a. CSH1206 (glucose) b: HF с. НI d. H2O2 e. LINO3 3. Give the Arrhenius and Bronsted definitions of acids and bases. Why are the Bronsted definitions more useful?arrow_forward3. 8.0 mL of HCI was added into a 10.0 mL graduated cylinder and weighed on a centigram balance. Looking at the photo to the right, record the mass to the nearest 0.01 Sost ha 12.71 gram in your data table (line 4)arrow_forwardMy teacher says the answer is 0.84 M, but how?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY