
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give answer as soon as possible please
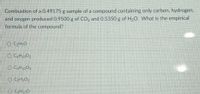
Transcribed Image Text:Combustion of a 0.49175 g sample of a compound containing only carbon, hydrogen,
and oxygen produced 0.9500 g of CO, and 0.5350 g of H2O. What is the empirical
formula of the compound?
O CH;O
O CH10O2
O CH102
O CH1,0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Review I Constants i Perlodic Table As a food chemist for a major potato chip company, you are responsible for determining the salt content of new potato chip products for the packaging label. The potato chips are seasoned with table salt, NaCl. You weigh out a handful of the chips, boil them in water to extract the salt, and then filter the boiled chips to remove the soggy chip pieces. You then analyze the chip filtrate for Cl- concentration using the Mohr method.First you prepare a solution of silver nitrate, AGNO3, and titrate it against 0.500 g of KCl using the Mohr method. You find that it takes 64.5 mL of AgNO3 titrant to reach the equivalence point of the reaction. You then use the same silver nitrate solution to analyze the chip filtrate in a Mohr reaction, finding that the solution yields a rusty brown precipitate when 45.4 mL of titrant is added. Part A If the sample of chips used to make the filtrate weighed 76.5 g , how much NaCl is present in one serving (105 g ) of chips?…arrow_forwardWhat is the product of the following reaction?arrow_forwardDashboard ← → C = x A ALEKS-Andrew Herrera - Learn x + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-lvgXwPgmUhvITCeeBZbufu BYTI0Hz7m7D3ZccVTgVx17hFt0cVC3NOPACUZOQGh6eoPJSZm2w-Wib... OTHERMOCHEMISTRY Using specific heat capacity to find temperature change 84°F Sunny A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 5.20 kg of water at 33.7 °C. During the reaction 84.5 kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J-g¹K -1 . answer to 3 significant digits. C Explanation Check -- X 5 Q Search: ▬▬▬▬▬▬▬▬▬▬▬▬▬▬ t LDUC H 0/5 ^ Round your G © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 4:33 PM 7/16/2023 D Andrew V F ? olo Ar Oarrow_forward
- ||| O NAMING AND DRAWING ORGANIC MOLECULES Naming normal alkanes Name these organic compounds: 43°F Mostly sunny Explanation structure H I H-C-H H H H H H H H 1 | | | | | | | -C. C-C H-C-C. | | H H Check | 1 I H H H | С-Н | H H н-с | H H H 1 -C-H X Q Search name Ś [***] 0/3 a Jessica V ? 0 000 B © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 8:50 PM 4/23/2023 نشاء +arrow_forwardCourse Home ← → C 3 My Courses Course Home Syllabus Scores eText Document Sharing User Settings Course Tools X b Answered: What Celsius temperat X ✰ openvellum.ecollege.com/course.html?courseld=17588559&OpenVellumHMAC=6d3aa364def4c39d3478d59efb4ace2f#10001 58°F Sunset coming Next > O x ⠀ 4:08 PM 11/13/2022arrow_forwardHow would you solve c? Transcribed Image Text:Temperature (°C) AN A student conducts an experiment to determine the value of for the dissolution of Na₂C₂H₂O₂ (s). The student dissolves 10.0 g of Na₂C₂H₂O₂ (s) in room-temperature water in a beaker and measures the temperature over time. The data are given in the graph above. 24- 22- 20 18 16 14 12- 10 Time (a) The student touches the side of the beaker after the dissolution has occurred and observes that it is cold. What experimental evidence is consistent with the student's observation? (b) Is the dissolution of endothermic or exothermic? Justify your answer in terms of the flow of energy between the system and surroundings. in kJ/mol. (c) The student calculated the energy change for the dissolution to be 4600 J. Based on this value, calculate AH solnarrow_forward
- For the arrhenius equation what are the other deviations for the formula like the ones i have to solve for t1 and k1.arrow_forwardModule 14 Discussion: Logarithmic Application A Radiative Forcing Radiative forcing, R, measures the influence of carbon dioxide in altering the additional solar ration trapped in Earth's atmosphere. The International Panel on Climate Change (IPCC) in 1990 estimated k to be 6.3 in the radiative forcing equation R = k In(), where Co is the preindustrial amount of carbon dioxide and C is the current level. (Data from Clime, W., The Economics of Global Warming, Institute for International Economics, Washington, D.C.) (a) Use the equation R = 6.3 In() to determine the radiative forcing R, in watts per square meter (W) to the nearest tenth, expected by the IPCC if the carbon dioxide level in the atmosphere doubles from its preindustrial level. (b) Determine the global temperature increase T, in degrees Fahrenheit to the nearest tenth, that the IPCC predicted would occur if atmospheric carbon dioxide levels were to double, given T(R) = 1.03R. Search entries or author Unread 96 4444 ↑ Oll 7…arrow_forwardSign in Week 15 Recitation Caleb Young Oklahoma - CHEM 1315 (Section 001) - Fall20 - ROCHER > Activities and Due Dates > Week 15 Recitation O Assignment Score: 50% Resources O Hint Check Answer Question 9 of 10 > When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this 2: reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH,OH(g) + 0,(g) → CO,(g) + 2H,O(1) AH = –764 kJ How much methanol, in grams, must be burned to produce 629 kJ of heat? 26.33 mass: TOOLS x10 about us careers privacy policy terms of use contact us help 5:14 PM 12/11/2020 50arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY