
Concept explainers
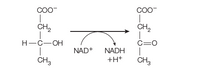
Freshly prepared mitochondria were incubated with β-hydroxybutyrate, oxidized cytochrome c, ADP, Pi, and cyanide. β-hydroxybutyrate is oxidized by an NAD+-dependent dehydrogenase.
The experimenter measured the rate of oxidation of β-hydroxybutyrate and
the rate of formation of ATP.
(a) Indicate the probable flow of electrons in this system.
(b) How many moles of ATP would you expect to be formed per mole of
β-hydroxybutyrate oxidized in this system?
(c) Why is b-hydroxybutyrate added rather than NADH?
(d) What is the function of the cyanide?
(e) Write a balanced equation for the overall reaction occurring in this
system (electron transport and ATP synthesis).
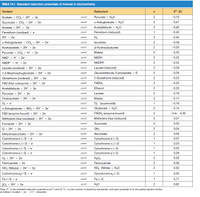
(f) Calculate the net standard free energy change (ΔG°') in this system,
using E'0 values from Table 14.1 and a ΔG°' value for ATP hydrolysis
of -32.2 kJ/mol.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

- When the antibiotic X is added to actively respiring mitochondria, several things happen: the yield of ATP decreases, the rate of O2 consumption increases, heat is released, and the pH gradient across the inner mitochondrial membrane increases. Does X act as an uncoupler or an inhibitor of oxidative phosphorylation? Explain the experimental observations in terms of the antibiotic’s ability to transfer K+ ions across the inner mitochondrial membrane.arrow_forwardAssume that 2.5 ATPs are generated per NADH and 1.5 ATPs per FADH2. How many ATPs are generated from the FADH2 and NADH molecules from each repetition of the ββbeta-oxidation pathway? Express your answer as an integer.arrow_forwardConsider the net summary equation for glycolysis. Suppose 13 molecules of glucose enter glycolysis. Calculate the number of molecules produced or used (a-d) upon completion of glycolysis utilizing all 13 glucose molecules. (a) # P; used (b) # pyruvates produced (c) # NADH produced (d) #ADP used.arrow_forward
- Cyanide is a rapidly acting, potentially deadly chemical that can exist in various forms. If accidentally ingested or inhaled, cyanide can cause rapid death by binding to complex IV (cytochrome oxidase) of the electron transport chain in the mitochondria. A.What is the mechanism by which cyanide stops cellular respiration? Be specific. B.Does cyanide cause an effect at the beginning or the end of the cellular respiration pathway? C.Does this make a difference on the effect that this chemical can have on our cells? Why? D.How does cyanide’s course of action affect the remainder of the cellular respiration pathway? E.If a person accidentally swallows cyanide, mention a potential treatment that is currently available. What is the mechanism of action of this treatment? Be specific. Please answer completely will give rating surely All questions answers neededarrow_forward(a) NAD+ kinase catalyzes the ATP-dependent conversion of NAD+ to NADP+. How many reducing equivalents are involved in this reaction? (b) How many reducing equivalents are involved in the conversion of ferric ion to ferrous ion? (c) How many reducing equivalents are involved in reducing one molecule of oxygen gas to water?arrow_forwardIodoacetate reacts irreversibly with the free -SH groups of cysteine residues in proteins. List which Calvin cycle enzyme(s) you would predict to be inhibited by iodoacetate, and briefly explain whyarrow_forward
- In oxidative phosphorylation, how many molecules of ATP are produced per molecule of NADH in the mitochondrion?arrow_forwardExplain why pyruvate does not enter the mitochondrion in the absence of oxygen?arrow_forwardThe cytochromes are heme-containing proteins that function as electron carriers in the mitochondria. Calculate the difference in the reduction potential (AE°') and the change in the standard free energy (AG°) when the electron flow is from the carrier with the lower reduction potential to the higher. cytochrome c₁ (Fe³+) + e¯ = cytochrome c₁ (Fe2+) E°' = 0.22 V cytochrome c (Fe³+) + e¯ = cytochrome c (Fe²+) E°' = 0.254 V Calculate AE°' and AG°'. AE°' = AG°' = V kJ/molarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





