
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
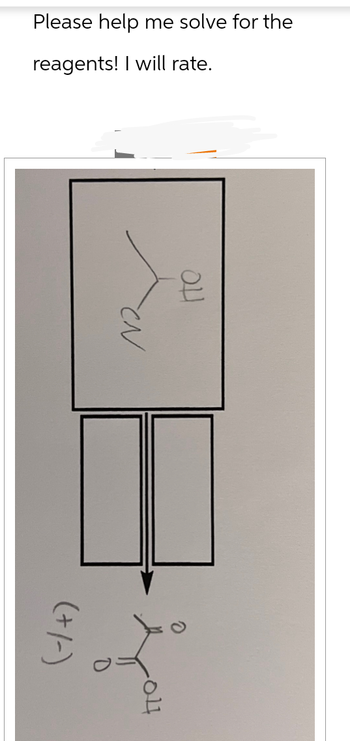
Transcribed Image Text:Please help me solve for the
reagents! I will rate.
วน
CN
0
под
(+/-)
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the volume in liters of a 1.7 x 10 mmol/L mercury(I) chloride solution that contains 700. mg of mercury(I) chloride (Hg,Cl,). Be sure your answer has the correct number of significant digits. x10 ?arrow_forward(4.21 x 10-9) x (3.2 x 10-6)= (4.1-0.0093) x (0.21 +0.19) = (4001 +0.043 +3.3) x 0.0001 = (6.5 x 10-5)/ (3.0 x 10-3)= (3.72 +8.35)x (6.231 x 102) / (3.000 x 104) =. (6.00 x 106) + (5.0 x 105) + (5 x 105) = (4.12 -3.91) x 6.124 1.57 x 0.5216arrow_forwardCalculate the number of millimoles (mmol) of methanol (CH3OH) in a 49.3 mL sample of methanol if, at this temperature, the density of methanol is 0.775 kg/m3. Enter at least 3 digits after the decimal point.arrow_forward
- (4.3 x 10^4) / (2.9 x 10^-3)arrow_forwardConvert 26.4in^3 to mLarrow_forwardthe carat(ct) is a unit of mass used for measuring gemstones and pearls. it is believed that the word comes from Greek for carob seed, because carbo seeds were used to measured jewelry throughout history. in modern times it has been using jewerly measurment, which of the following set up will give the correct converstion for 1.00 troy ounce (t oz) of gold to carat (ct) 24 (g)=1 (dwt) 20(dwt)=1 troy ounce(t oz) 12 (t oz) = 1 troy pound (lb t) 1 (g) =0.0648 (g) 1 carat (ct) =0.200 (g) a) 1.00 t oz=1lb t=12 toz=20dwt=24g=1ct b) 1 toz=1dwt=24g=1gr=20dwt=1lbt=1lb t=1ct c)1.00 t oz=20dwt=24g=1/0.200 ct c)1.00 toz=24g=1gr=1ctarrow_forward
- How many milligrams of mercury(I) chloride (Hg2Cl2) must be present in a 362 mL sample of water to give 5.70 ppm (m/v) of Hg2Cl2? Place your answer in the box. Express your answer using only a number without text. Report your result in decimal notation and to the proper number of significant figures. All numbers are measured.arrow_forwardWhat do the abbreviations in the equations below mean? (write next to the equations) C + MU = R C% + MU% = 100% MU% = MU × 100% R C% = C × 100% Rarrow_forwardThe first onearrow_forward
- Can you show your math? 10^-6 times 500 = .0005 and that divided by 1.07 is not 0.0035?arrow_forwardA student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. (84. cm )· 0= ? m² 3 3arrow_forwardPLEASE SEE IMAGE ATTACHEDarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY