
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
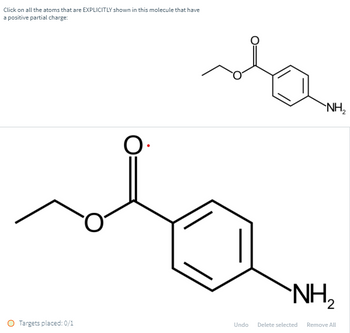
Transcribed Image Text:Click on all the atoms that are EXPLICITLY shown in this molecule that have
a positive partial charge:
O.
`NH₂
Targets placed: 0/1
`NH₂
2
Undo Delete selected Remove All
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer the following questions about the Lewis structure for calcium hydrogen carbonate, Ca(HCO3)2 There are calcium ions and hydrogen carbonate ions. There are ionic bonds in the molecule. The calcium ion has electron dots. Each hydrogen carbonate ion has central atoms and valence electrons. Each hydrogen carbonate ion has single covalent bonds, double covalent bonds, and triple covalent bonds. In each hydrogen carbonate ion, the central C has REDS, the shape is y and the bond angles are about In each hydrogen carbonate ion, the central O has REDS and the shape is а. О b. 1 С. 2 d. 3 е. 4 f. 5 g. 8 h. 10 i. 12 j. 14 k. 16 I. 18 m. 20 n. 24 О. 26 р. 30 q. 32 r. 34 S. 36 t. diatomic u. pyramidal v. linear z. bent y. trigonal planar w. monoatomic ions x. tetrahedral aа. 1200 bb. 180° Cc. no bond angles, no central atom nonpolar dd. 109.5° ее. polar ff. gg. ionic hh. O and Oarrow_forwardDecide whener each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion CS2 H Br CH₂ CI polar or nonpolar? polar nonpolar polar nonpolar polar nonpolar atom closest to negative side 0arrow_forwardwww TT In the first column type the number of atoms in the covalent compound. In the second column, type the total number of VALENCE electrons in the compound (all electrons: shared and unshared electrons). In the third column, determine the total number of possible chemical bonds in the compound. Compound H₂ Cl₂ 0₂ N₂ H₂O SF2 CH4 NH3 PF3 CC14 C₂H6 CHEMISTRY Covalent Compounds and Electrons Number of Atoms I Total Valence Electrons Total possible of chemical bondsarrow_forward
- Draw a Lewis structure for the molecular formula: NOCl This molecule only contains nitrogen, oxygen, and chlorine atoms. All atoms in this molecule should have formal charges of zero. What is the geometry of the electron groups around oxygen?arrow_forwardDecide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion H Br CO2 CCI polar or nonpolar? polar O nonpolar O polar O nonpolar O polar O nonpolar X atom closest to negative side 0 0 0 Sarrow_forwardDecide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion F₂ H₂O CHA polar or nonpolar? O polar O nonpolar O polar O nonpolar O polar O nonpolar X atom closest to negative side 0 Sarrow_forward
- Decide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion HCN CH₂ CI 1₂2 polar or nonpolar? polar nonpolar polar nonpolar polar nonpolar X atom closest to negative side 0 Śarrow_forwardWhat is the name of the ionic compound CaCO3? Enter the name in all lowercase letters (including any roman numberals) with no spaces after the names. What is the name of the ionic compound AgBr? Enter the name in all lowercase letters (including any roman numberals) with no spaces after the names.arrow_forwardWhat is the name of the ionic compound FeCl3? Enter the name in all lowercase letters (including any roman numberals) with no spaces after the names. What is the name of the ionic compound KMnO4? Enter the name in all lowercase letters (including any roman numberals) with no spaces after the names.arrow_forward
- Answer the following question.arrow_forwardDecide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HC1 and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion HCN CH 4 HI polar or nonpolar? polar nonpolar polar nonpolar polar nonpolar atom closest to negative sidearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY