
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Select all of the statements that are supported by the diagram shown below:
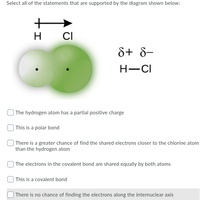
Transcribed Image Text:Select all of the statements that are supported by the diagram shown below:
H
CI
8+ 8-
H-CI
| The hydrogen atom has a partial positive charge
| This is a polar bond
| There is a greater chance of find the shared electrons closer to the chlorine atom
than the hydrogen atom
| The electrons in the covalent bond are shared equally by both atoms
| This is a covalent bond
| There is no chance of finding the electrons along the internuclear axis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A liquid that does not conduct an electrical current although it does dissolve in water is most likely… a) a polar molecular compound b) a nonpolar molecular compound c) an ionic compound d) a metallic substance e) Both c and d are correctarrow_forwardPlease answer the second question I need itarrow_forwardWhat happens to the ion atoms when sodium and chlorine mix to make sodium chloridearrow_forward
- Need help with part A and Barrow_forwardIn which form of water are the water molecules closest together? (A) liquid water, (B) ICE, (C) Water vaporarrow_forwardUse your knowledge of the correct number of covalent bonds to predict the formula for a simple compound formed between silicon and the following elements. (Enter NONE if no compound is likely to form.) (a) hydrogen chemPad Help (b) iodine chemPad Help (c) helium chemPad Help (d) seleniumarrow_forward
- A hypothetical metal has the simple cubic crystal structure shown in Figure 3.3. If its atomic weight is 84.2 g/mol and the atomic radius is 0,146 nm, compute its density. B/cm²arrow_forwardQuestion 34 of 50 > 0.5 / 1 View POIICIES Show Attempt History Current Attempt in Progress Mercury, water, and bromine are liquids at standard temperature. Their molar entropies are in the sequence H2O < Hg < Br2. Incorrect. Using molecular properties, explain why bromine is more disordered than mercury. Select those that apply. Bromine is diatomic. Mercury is monoatomic. Diatomics are more ordered than monoatomics. Water has strong hydrogen bonding interactions. O Hydrogen-bonded substances are less ordered than substances without hydrogen-bonds.arrow_forwardWhat is the term for a homogenous mixture of two or more metals? A) metalloid B) a compound C) an alloy D) malleable E) none of the abovearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY