
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Complete these nucleophilic substitution reactions. In each reaction, show all
electron pairs on both the nucleophile and the leaving group.
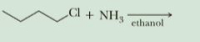
Transcribed Image Text:Cl
+ NH3
ethanol
Expert Solution
arrow_forward
Step 1
These nucleophilic substitution reaction has to be completed.
All electron pairs on both the nucleophile and the leaving group has to be shown.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can we determine whether the equilibrium will favor products in a nucleophilic substitution?arrow_forwardGood nucleophiles that are weak bases favor substitution overelimination. Explain this ?arrow_forwardLabel all of the nucleophilic and electrophilic sites on the following molecule НО. NH₂arrow_forward
- Identify the nucleophile and leaving group and draw the products of attached substitution reaction.arrow_forward+ In an SN2 reaction, a lone pair in the of the electrophile. A) a orbital; o orbital B) non-bonding orbital; o* orbital of the nucleophile reacts with the C) non-bonding orbital; o orbital D) TT orbital; σ orbital E) TT orbital; o* orbitalarrow_forwardDetermine which would be more reactive with a nucleophilearrow_forward
- Determine whether the following reaction is an example of a nucleophilic substitution reaction: O" Molecule A + NO₂ Molecule B NO₂ + Harrow_forwardPlease explain in detail idk how to go about this problem.arrow_forward2. Draw a mechanism for the reaction of the nucleophile and the electrophile as indicated below. Also draw the product produced. CH3 H3C+CI CH3 + HOarrow_forward
- This reaction does not correctly highlight the nucleophile because... + HCI A) In elimination reactions the alkene is always the electrophile B) In elimination reactions the alkene is always the nucleophile C) In electrophilic additions to alkenes the alkene is always the electrophile D) In electrophilic additions to alkenes the alkene is always the nucleophilearrow_forwardWhich is/are NOT TRUE about bimolecular nucleophilic substitution reactions? Select one or more: 1. A carbocation intermediate is formed. 2. A strong nucleophile displaces a halogen atom in a concerted mechanism. 3. Presence of polar aprotic solvents promotes this reaction. 4. Methyl halides react faster than secondary alkyl halides.arrow_forwardThe reaction of an alkyl chloride with potassium iodide is generally carried out in acetone to maximize the amount of alkyl iodide that is formed. Why does the solvent increase the yield of alkyl iodide? (Hint: Potassium iodide is soluble in acetone, but potassium chloride is not.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY