
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Circle and label the hemiacetal
following carbohydrates:
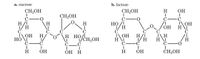
Transcribed Image Text:а. sucrose
b. lactose
CH2OH
CH2OH
H
ОН
CH2OH
C
НО /1
i/H
C
I OH
H \i
C
/OH H
C
Пон
H/!
1/ H
C
Пон
НО \
C
H
H
C
H HO/!
C
I\H
H \I
C
H
H/I
1%CH,OH
H
CC
-C
H.
ОН
ОН
H
H
OH
CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Give the omega-n designation for each acid. Part 1 of 2 stearidonic acid CH3CH2(CH=CHCH2) 4 (CH2)2COOH omega- acid Part 2 of 2 linoleic acid CH3(CH2) CH=CHCH2CH=CH(CH2), COOH omega- acidarrow_forwardName the following fatty acids by the symbol (C:B)arrow_forwardConsider this monosaccharide. H H OH H .OH HO H HO H CH₂OH Part: 0/3 Part 1 of 3 Label the chirality center(s) for the monosaccharide by highlighting the carbon(s) in the structure below. H H -OH H -OH HO -H HO -H CH₂OH હેarrow_forward
- Fill in ..... (1) the complete systematic names for sugars A, B and C (remember to you pyranosyl or furanosyl because these are not the terminal sugar). (2) The appropriate descriptor of the glycosidic bonds where appropriate/necessary. (3) Draw the missing sugars as indicated onto the given diagram. HO OH GED A HO OH a-L-psicofuranose (1➜6)- (1➜4)- a-D-idopyranose HO OH B OH OH OH OH SUGAR A SUGAR B SUGAR Carrow_forwardFor the following lipid, answer the questions listed below. 0- HC-o (CH)),CH=CH(CH,);CH; H,C-o-c-(CH2);CH=CHCH,CH=CHỊCH,),CH, Is this lipid considered a fat or oil? a. b. How many H2 molecules would be needed to go through a complete hydrogenation reaction? Would the hydrogenated product (after hydrogenation has occurred) have a higher or lower mp than the original starting material? С.arrow_forwardWhich of the following monosaccharides structural relationship is CORRECT? * CHO CHO CHO но- H- HO -- но -H- но -OH- H- но -- H- H- H- OH но- -H- ČH2OH ČH,OH CH,OH 1 3 1 and 2 are enantiomers, while 2 and 3 are diastereomers 1 and 2 are diastereomers, while 2 and 3 are diastereomers 1 and 2 are enantiomers, while 2 and 3 are enantiomers O 1 and 2 are diastereomers, while 2 and 3 are enantiomersarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON