
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
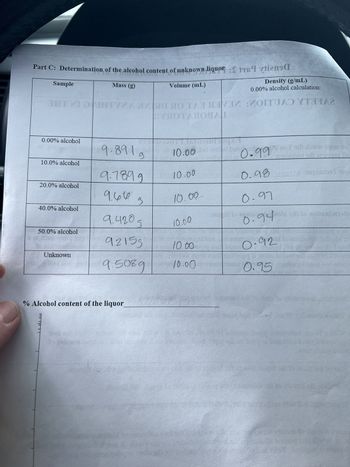
Transcribed Image Text:Part C: Determination of the alcohol content of unknown liquor:
Volume (mL)
Sample
ТИШТИНА
0.00% alcohol
10.0% alcohol
20.0% alcohol
40.0% alcohol
50.0% alcohol
Unknown
Mass (g)
1o podina ko
9.8919
9.7899
9.600
9
9.420 д
% Alcohol content of the liquor
9.2155
9.5089
2VLELA CVOLION EAEK EVI OK DRA
IVBOKY LOBAN
ocen
10.00
10.00
10.00
10.00
10.00
10.00
tiend
Density (g/mL)
0.00% alcohol calculation:
ися
→
о
0.99 во
0.99
Ino7 sm dole 120.00
supinubst sdi
na
LANG
411
be: [scordas:
0.97
0.94 in oil to notisalarisi
0.92
0.95
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Water Sugar Lemon Juice Lemonade Percent Leftover Yield Ingredients 946.36 g 196.86 g 193.37 g 719.84 g Sugar and water Just Lemons Lemonade Recipe Equation: 2 water+ sugar + lemon juice = 4 lemonade Mole conversion factors: 1 mole of water = 1 cup = 236.59 g %3D 1 mole of sugar = 1 cup = 198 g %3D %3D 1 mole of lemon juice = 1 cup = 229.96 g %3D %3D 1 mole of lemonade = 1 cup = 225.285 g %3D Show your calculations below.arrow_forwardA student prepares a 0.28 mM aqueous solution of acetic acid (CH,CO,H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. |% x10arrow_forwardBased off information from the attachment, please help with #9 on second attachmentarrow_forward
- PERCENT WATER IN A HYDRATE DATA/CALCULATION SHEET NAME 1.. crucible, cover, BaCl2 2H2O _\8, a0G! g crucible and cover 14.0188g BaCl 2H2O 4.1873 g first second third weighing weighing weighing crucible, cover, anhydrous BaCl, 7.5824 17.5815 H2O lost (use your last weighing) 17.58158 % water, experimental SHOW CALCULATION % water, theoretical (See prelab) % error SHOW CALCULATIONarrow_forwardWine goes bad soon after opening because the ethanol (CH3CH₂OH) in it reacts with oxygen gas (0₂) from the air to form water (H₂O) and acetic acid (CH₂COOH), the main ingredient of vinegar. What mass of water is produced by the reaction of 8.9 g of ethanol? Round your answer to 2 significant digits. 08 g x10 ? Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility Explanation Check X MacBook Pro & Xarrow_forwardFrom the graph of temperature vs. volume data from a fractional distillation of 100 mL methanol-water mixture, What is the ratio of water:methanol (mL:mL)? 100 90 Temp 80 (*C) 70 60 10 20 30 40 50 60 70 80 90 100 Volume (mL) Selected Answer: 67:33arrow_forward
- What is the mass percent of 60 g NaCl in a 200-g solution? Express your percent as a decimal.arrow_forwardWhat is the Mass of solvent in grams? Add together the masses of the liquid water and ice added to the calorimeter. Convert the volume of liquid water to mass using 1.000 g/mL as the density of water (see procedure for volume used). Side note:Molarity of the solution is 0.76612903225806arrow_forwardData obtained: Chips # of extractions Chips' weight (g) Fat weight (g) Regular 3 20.043 6.745 3 20.187 6.438 3 20.198 7.451 Low fat 3 19.456 3.982 3 20.072 4.547 3 20.192 4.589 Mass percent is a method of expressing the concentration of a substance in a mixture or element in a compound. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. The formula is: mass % of component= (mass of component / total mass) x 100% 1) Reproduce the following table, and use the data to determine the mass % of fat in the chips for each trial. 2) Show one example calculation. Chips Trial % by mass of fat Regular 1 2 3 Lowfat 1 2 3arrow_forward
- Z @ ▶ 12. ✿ Text-to-Speech You are asked to make 43.0 grams of iron (Fe) from iron III oxide (Fe:O) and carbon monoxide (CO) as shown in the chemical equation below. Fe₂O3 + 3C0→2Fe + 3C0₂ How many grams of iron (III) oxide must you use? OA. 61.5g OB. 122 g OC. 0.777 g OD. 686 g 1246 2 W S # X 15 # 3 E 14 H L $ 4 Q Search R D F % 5 T CV 6 b VI B | & Y lipi GH 7 Lo *K U N 8 J 19 144 9 K M fo Dl O In DDI P : [ = ? 1 F 10 10 10 10 1arrow_forwardMasses of compounds used and observations NaCI CuSO4-5H20 Bright blue crystaline solids (NH4)2Fe(SO4)2 pale, Blue-green crystalline solid colorless Crystals Appearance of solid Mass of vial and 12.8364 12.7398 12.9445 compound (g) Mass of emptied vial (g) 11.6023 11.4888 11.9221 Mass of compound used (g) 1.2341. 1.2510. 1.0224 Volume of prepared solution = Type response here. Mass of sodium chloride used = Type response here.arrow_forwardA student prepares a 3.4M aqueous solution of propionic acid (C₂H₂CO₂H). Calculate the fraction of propionic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % x10 X 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY