
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
predict the major products
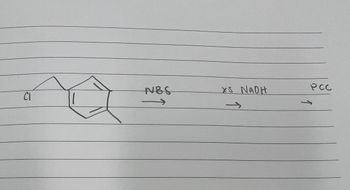
Transcribed Image Text:CI
NBS
XS NaOH
→>>>
-
РСС
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Good hand written explanation ASAP What will be the character following salts are acidic, basic, or neutral at 25°C: (a) Cu(CH3COO)2; (b) NH4F; (c) KHC6H6O6. a) (a) acidic; (b) acidic; (c) acidic b) (a) acidic; (b) acidic; (c) basic c) (a) acidic; (b) basic; (c) basic d) (a) basic; (b) basic; (c) basicarrow_forward10.0 cm³ of a solution of a strong acid with a pH of 3 is added to a volumetric flask and the total volume is made up to 1.00 dm³ by adding distilled water. The resulting solution is then thoroughly mixed. What is the pH of the diluted solution?arrow_forwardWhich of these is not a conjugate acid-base pair? O NaH,PO4 ; Na2HPO, O HCIO,; HCIO, O H;O*; H2O O CH3CH,NH;C; CH;CH,NH2arrow_forward
- (a) Wr ite an equat ion for the react ion in whichH2C6H7O5-(aq) acts as a base in H2O(l). (b) Write an equationfor the reaction in which H2C6H7O5-(aq) acts as an acidin H2O(l). (c) What is the conjugate acid of H2C6H7O5-(aq)?What is its conjugate base?arrow_forward8arrow_forwardWrite the chemical equation and the Kb expression for thereaction of each of the following bases with water: (a) dimethylamine,(CH3)2NH; (b) carbonate ion, CO32-; (c) formateion, CHO2-.arrow_forward
- 7. (a) Predict whether, KCN, potassium cyanide will form an acid, base or neutral solution. (b) Calculate the pH of a 0.500 M solution of KCN. K, for HCN is 5.8 x 10-". 14 INQarrow_forwardPlease answer both asapparrow_forwardGive clear detailed Solution with explanation needed....don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Which of the following reactions is not product-favored? ONAOH(aq) → Na*(aq) + OH (aq) NH3(aq) + H2O() → NH4*(aq) + OH-(aq) HCIO4(aq) + H20() → H30*(aq) + CIO4-(aq) O HCI(aq) + H20() → H30*(aq) + CI-(aq) H2SO4(aq) + H20() → H3O*(aq) + HSO4-(aq)arrow_forwardWhich of the following is false regarding the reaction below? H2SO,(aq) + H20() = HSO, (aq) + H;O (aq) O H,SO3(aq) acts as an acid in this reaction. O HSO, (aqg) is the conjugate base in this reaction. O H,O (aq) and H,0() is a conjugate acid-base pair. O 1,0(1) acts as an acid in this rçaction. 4 Previous Discussion veek...docx dayum.pdf PDF t (9) docx BluebeardBrothers..pdf POF ype here to searcharrow_forward5. Which one of the following equations would make more products upon addition of acid (H)? Briefly explain. H,SO,(aq) 2 HSO, (aq) + H (aq) HCl(aq) 2H(aq) + Cl'(aq) NH, (aq) + OH(aq) 2 NH;(aq) +H,O(1) 2 Fe,0;(s) 2 2 FeO(s) + O>(g) H(aq) + NH;(1)2 NH, (aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning