
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
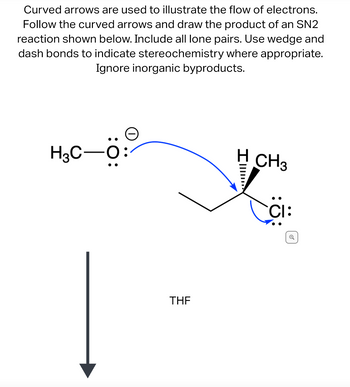
Transcribed Image Text:---
**Electron Flow in SN2 Reaction Mechanism**
Curved arrows illustrate the flow of electrons in an SN2 reaction, providing insight into the mechanistic steps. Follow these arrows to predict the reaction's product. The diagram requires the inclusion of all lone pairs, and the use of wedge and dash bonds for indicating stereochemistry is essential. Inorganic byproducts should be ignored for clarity.
**Diagram Explanation:**
- The left structure shows a methoxide ion (CH₃O⁻) with three lone pairs on the oxygen.
- A blue curved arrow originates from the negatively charged oxygen and points towards the carbon atom bonded to chlorine, indicating a nucleophilic attack.
- The right structure depicts a secondary carbon center bonded to a hydrogen and methyl group, where chlorine is the leaving group. The chlorine has three lone pairs and is negatively charged as it leaves.
- The use of wedge (solid) and dash (hashed) bonds shows the stereochemistry around the chiral center.
- An arrow labeled "THF" points downward, suggesting tetrahydrofuran is the solvent.
This detailed representation aids in understanding electron flow and stereochemical changes in SN2 reactions.
---
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
whats the final products
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
whats the final products
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product(s) from addition of HBr to the alkene below. O • You do not have to consider stereochemistry. If there is more than one major product possible, draw all of them. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. . CHCH3 **** AHRRA ChemDoodlearrow_forwarddraw the organic product of the reaction. be sure to include lone pairs and formal charges on structures, and include sterochemistry if revelant.arrow_forwardDraw a structural formula for the major organic product of the reaction shown. CH3 CHCH₂CCH3 CH3 You do not have to consider stereochemistry. • If there is more than one major product possible, draw all of them. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ? + HI OOD ChemDoodleⓇ Sn [F <arrow_forward
- 4. A hydrocarbon of unknown structure (ooh, you're gonna LOVE OCII!!!) has the molecular formula C3H10. When treated with rhodium during catalytic hydrogenation, three molar equivalents of hydrogen are absorbed. When this unknown hydrocarbon is treated with CACO3 and H2/Rh, only ONE molar equivalent of hydrogen is absorbed. a. Draw two structures for this unknown that fits the data given. b. Are there rings present? c. Any double/triple bonds? d. How many degrees of unsaturation does this unknown have? e. Could it possess a BENZENE ring, which IS unsaturated AND has a RING...arrow_forwardSee image belowarrow_forwardDraw the two major products obtained in the reaction shown. Br2 (one equivalent) CH2Cl2 •You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the product of an SN2 reaction shown below. Include all lone pairs. Use wedge and dash bonds to indicate stereochemistry where appropriate. Ignore inorganic byproducts.arrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Complete the following reaction by drawing the structure of the major product(s) expected. CH3 HCl CH3C=CH2 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • If there is more than one major product possible, draw all of them. Separate multiple products using the + sign from the drop-down menu. P. opy aste IF ChemDoodle 9 more group attempts remaining Submit Answer Retry Entire Grouparrow_forwardOCCH, [Review Topics] [References] Draw a structural formula for the substitution product of the reaction shown below. F + - CH3 OCCH3 Na CH;CO,H • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu. n [F ChemDoodle Cengage Learning | Cengage Technical Support MacBook Air 80 000 DII DD F4 F5 F6 E7 F8 F10arrow_forward
- Draw the products of the following reactions, indicating both regiochemistry and stereochemistry when appropriate. CH3 KMNO4 H30* `CH3 • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. P opy aste ? ChemDoodle® >arrow_forward[Review Topics] [References] An alkene having the molecular formula C6H10 is treated sequentially with ozone (03) and zinc/acetic acid to give the product/s shown. CH3CCH,CH,CH2CH Draw a structural formula for the alkene. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. P. aste Ado ChemDoodle Previous Nextarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Draw a structural formula for the missing product in the following reaction. CH3CH2CHC-OH + NaHCO3 H20 ? + CO2 + H20 CH2CH3 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Include cationic counter-ions, e.g., Nat in your answer, but draw them in their own sketcher. C opy aste C. CH4 ChemDoodle Submit Answer Retry Entire Group 4 more group attempts remaining Previous Nex Email Instructor Savearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY