
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
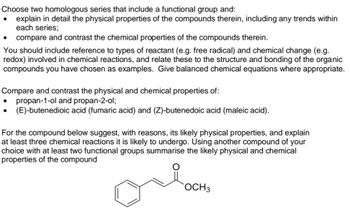
Transcribed Image Text:Choose two homologous series that include a functional group and:
explain in detail the physical properties of the compounds therein, including any trends within
each series;
compare and contrast the chemical properties of the compounds therein.
You should include reference to types of reactant (e.g. free radical) and chemical change (e.g.
redox) involved in chemical reactions, and relate these to the structure and bonding of the organic
compounds you have chosen as examples. Give balanced chemical equations where appropriate.
●
●
Compare and contrast the physical and chemical properties of:
propan-1-ol and propan-2-ol;
(E)-butenedioic acid (fumaric acid) and (Z)-butenedoic acid (maleic acid).
●
For the compound below suggest, with reasons, its likely physical properties, and explain
at least three chemical reactions it is likely to undergo. Using another compound of your
choice with at least two functional groups summarise the likely physical and chemical
properties of the compound
ore
OCH 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hw.22. With full explanation.......arrow_forwarda) Choose two homologous series that include a functional group and explain in detail the physical properties of the compounds therein, including any trends within each series. b) For the two homologous series you have chosen, compare and contrast the chemical properties of the compounds therein. Include reference to types of reactants (e.g. free radical) and chemical change (e.g. redox) involved in chemical reactions, and relate these to the structure and bonding of the organic compounds you have chosen as examples. Give balanced chemical equations where appropriate. [AC2.2] oturalarrow_forwardGive detailed Solution with explanation needed with structure. don't give Handwritten answerarrow_forward
- Answer Q18, 19, 20, 21arrow_forwardCompound K, L and M are three isomers with the molecular formula C5H10O.Compound K cannot be oxidized, while compound M and L can be oxidized.Oxidation of compound L with hot acidified potassium permanganate,KMnO4 solution yields 2-methylbutanoic acid. When treated with Iodoformreagent, yellow precipitation only occurs in compound K. Fehling test onlyyielded positive results for compound L and negative for compound M andK. Compound M is then reacted with hydrogen chloride, HCl produceschlorocyclopentane (i) Draw the structural formula of compounds K, L and M.arrow_forwardcomplete the reactiona and draw it outarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY