
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Choose the set of letters corresponding to the BEST answer.
-gives two moles of QRS upon hydrolysis
-the most reactive towards nucleophilic acyl substitution
-can be formed from the alcoholysis of TUV
-has the most acidic proton
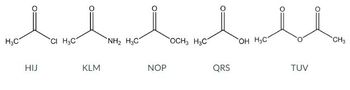
Transcribed Image Text:H₂C
HIJ
CI H3C
KLM
NH, HỌC
NOP
OCH3 H3C
QRS
OH H₂C
TUV
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with the following electrophile followed by acid workup when relevant: CN Modify the provided structures of diethyl malonate and the given electrophile to draw the product formed. Note: the eraser tool can be used to erase bonds, and atoms can be moved by selecting them with the selector tool and then dragging the selected atom(s) to a new position. If you make a mistake, you can use Ctrl-Z or the Undo tool. EtO H3C CN Edit Drawing OEtarrow_forwardHow is the mechanism for oxymercuration/demercuration reactions of alkenes understood? How are the (Markovnikov) products predicted and what reagents would you need to use to incorporate this reaction into a multistep synthesis strategy. Finally, what makes this a potentially more useful way to synthesize alcohols from alkenes than simple acid hydration?arrow_forwardEnamines can serve as enolate surrogates in reactions at the a-carbon. In the reaction sequence, the structures of the enamine addition product – the initial zwitterion and its neutral tautomer – are shown. Draw the structures of the two reactants forming these intermediates, and draw the structure of the final product, obtained via hydrolysis of the neutral intermediate. initial zwitterionic intermediate neutral intermediate tautomerization Reactants H,0 hydrolysis product Draw the two reactants. Draw the hydrolysis product. Select Draw Rings More Erase Select Draw Rings More Erase H Harrow_forward
- 58) Draw the product resulting from the following reaction. Indicate any relevant stereo chemistry. Eto EtOHarrow_forwardGive the major organic product of the following reaction sequence.arrow_forwardshawl the detailed beland the neaction Label each спе of the reaction the ardor listed belanu. of part of the defalled mechanism wing of the terms listed.. - ester hydrolysis decarboxylaton Keto- endi tautomensin reaction. mechanism proceeds in •OCN3 OH 1130 + он '+' CH 3 CH + CO₂arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY