
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Synthesize the following compound from butanoic acid and any other organic or inorganic compound containing 7 or less carbons
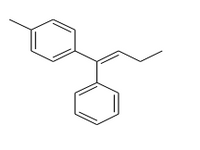
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Parts A,B&C Please.arrow_forwardPhenylethanol can be oxidised to phenylethanal or phenylethanoic acid, depending on the reagents used (both the alcohol and the aldehyde are of interest for their antimicrobial properties, while the acid is used to treat type II hyperammonemia): A (a) (b) (c) CoH,CH,CHO phenylethanal B C6H5CH₂CH₂OHC₂H₂CH₂CO₂H phenylethanol Suggest reagents (shown as A and B in the scheme above) that could be used to carry out the oxidation of the alcohol to the aldehyde and the acid, respectively. C6H5CH₂- Suggest two other syntheses of phenylethanoic acid, in each case indicating the starting materials and other reagents required, but not giving details of mechanism. One of your proposed syntheses must start with a compound which only contains seven carbon atoms (the acid product contains eight carbon atoms). phenylethanoic acid Phenylethanal can be converted to a hydrate in the presence of aqueous acid, though the position of equilibrium is very far to the left: H H+/H₂O OH C6H5CH₂-C-H OH Explain why…arrow_forward2. Write the structural formulas for the two most stable enol isomers of the following B carbonyl compounds.arrow_forward
- 22:25 Worksheet 2 chemistry Worksheet 2: Formulae and structures of organic compounds Complete the table below to identify the general formulae and structures of the organic compounds. Give reasons why. Name Homologous series Structural formula General formula (homologous series) Propan-1-ol Alcohol Reasons Propan-2-ol Reasons 2-methypropan-2-ol Worksheet 2: Formulae and structures of organic compounds Reasons 2-Bromopropane Reasons Propanone Reasons | 1-Propanal Reasons ج ال..arrow_forwardDraw the structures of the following aldehydes / ketones: 1.) 3-chloro-3-methylhexanal 2.) 3,4-diethyl-3-methylhexan-2-one 3.) 5-bromo-3-methyheptanalarrow_forwardGive the IUPAC name for the following compound. CH3-CH;-CH,-C-0-CH-CH, ethyl propanoate 4-hexanal ethyl propyl ether ethyl butanoate hexanoic acid « Previousarrow_forward
- I know there is: 1. Carboxylic group 2. Ester. 3. Ortho-disubstituted phenyl. But my answer is still partial.arrow_forwardDraw a structural formula for the major organic product of the following reaction: CH3- -CH=CHCOH + Cl₂ CH₂Cl2arrow_forwardQuestion 15.b of 25 Classify and describe the properties of the following nitrogen containing compound. Provide a systematic name for this structure. 2- N,N,N- 0 1- N- meth n- prop CH3 Ell -Z tri di tert- sec- hex eth pent W N H W N,N- 4- but +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY